
 Copy to clipboard
Copy to clipboard 
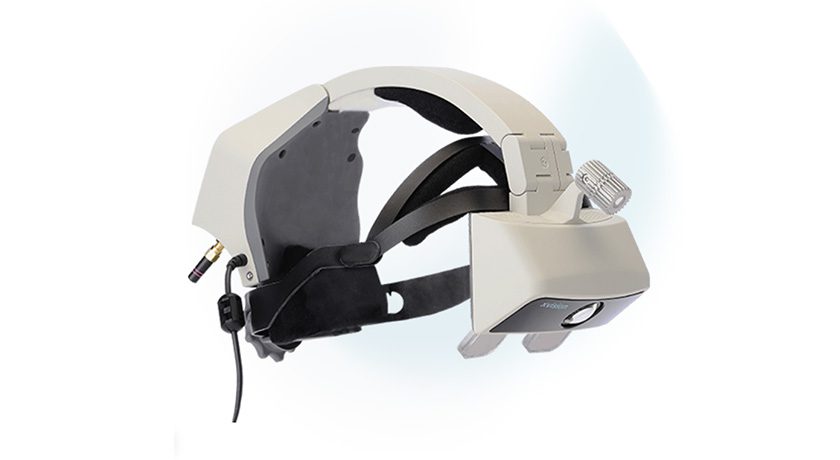
Augmedics received FDA 510(k) clearance of a new registration method for its xvision Spine System. The xvision CT-to-fluoroscopy (CT-Fluoro) registration method allows surgeons to navigate off a preoperative CT scan and fluoroscopic images from a standard 2D C-arm, eliminating the need for 3D intraoperative imaging. Augmedics also shared the successful completion of the first surgical case using its new CT-Fluoro registration.
Augmedics is conducting a limited launch of xvision’s CT-Fluoro registration at select sites through the end of 2024 and plans a full commercial launch to the U.S. market in Spring 2025.
The foundations of Augmedics’ CT-Fluoro registration application rest on an expansion of the company’s proprietary artificial intelligence capabilities launched in Spring 2023. CT-Fluoro also builds on Augmedics’ open platform with additional imaging and registration flexibility, allowing surgeons to choose between pre-op CT and intraoperative 3D imaging and registration.
“Our mission at Augmedics is to bring the proven benefits of navigation to as many surgeons and as many patients as possible. By removing the 3D imaging requirement, we’re removing a major barrier to adoption,” said Gwen Watanabe, Augmedics Interim CEO. “CT-Fluoro enables surgeons to unlock the benefits of AR navigation for patients in any facility.”
Source: Augmedics
Augmedics received FDA 510(k) clearance of a new registration method for its xvision Spine System. The xvision CT-to-fluoroscopy (CT-Fluoro) registration method allows surgeons to navigate off a preoperative CT scan and fluoroscopic images from a standard 2D C-arm, eliminating the need for 3D intraoperative imaging. Augmedics also shared the...
Augmedics received FDA 510(k) clearance of a new registration method for its xvision Spine System. The xvision CT-to-fluoroscopy (CT-Fluoro) registration method allows surgeons to navigate off a preoperative CT scan and fluoroscopic images from a standard 2D C-arm, eliminating the need for 3D intraoperative imaging. Augmedics also shared the successful completion of the first surgical case using its new CT-Fluoro registration.
Augmedics is conducting a limited launch of xvision’s CT-Fluoro registration at select sites through the end of 2024 and plans a full commercial launch to the U.S. market in Spring 2025.
The foundations of Augmedics’ CT-Fluoro registration application rest on an expansion of the company’s proprietary artificial intelligence capabilities launched in Spring 2023. CT-Fluoro also builds on Augmedics’ open platform with additional imaging and registration flexibility, allowing surgeons to choose between pre-op CT and intraoperative 3D imaging and registration.
“Our mission at Augmedics is to bring the proven benefits of navigation to as many surgeons and as many patients as possible. By removing the 3D imaging requirement, we’re removing a major barrier to adoption,” said Gwen Watanabe, Augmedics Interim CEO. “CT-Fluoro enables surgeons to unlock the benefits of AR navigation for patients in any facility.”
Source: Augmedics

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







