
 Copy to clipboard
Copy to clipboard 
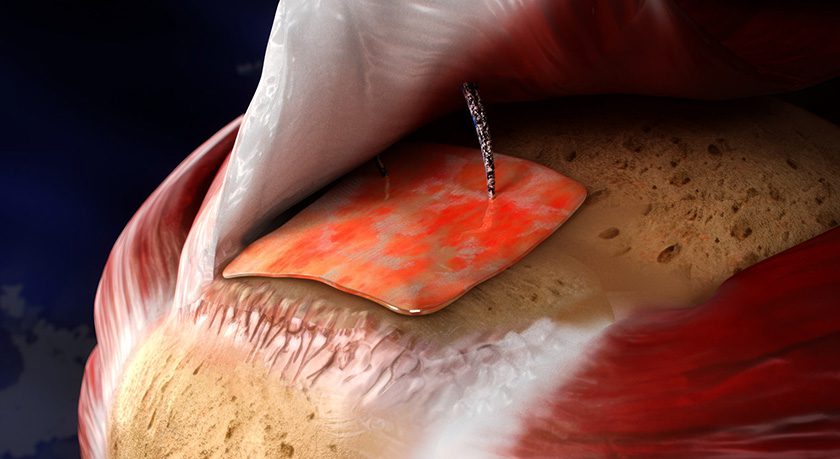
Atreon Orthopedics announced the milestone of the successful use of its ROTIUM Bioresorbable Wick in over 10,000 rotator cuff repair surgeries. The implant, which was granted initial FDA 510(k) clearance in 2019, is available in the U.S., Taiwan and other select countries worldwide.
The ROTIUM implant is a fully resorbable, synthetic-nanofiber scaffold designed to optimize healing at the critical tendon/bone interface in rotator cuff repairs. By supporting the body’s natural healing processes and promoting better tissue integration, ROTIUM can help surgeons achieve stronger, more consistently durable repairs.
The technology’s rapid adoption in the market may be attributed to three key factors:
- Data-Driven Efficacy & Safety – Proven results that support positive patient outcomes.
- Cost-Effectiveness – Affordable without compromising quality, appealing to price-sensitive markets.
- Ease of Use – A simplified approach to implantation that enhances surgical efficiency.
The ROTIUM technology is the culmination of over 16 years of research in tissue remodeling, utilizing Electrospun engineered scaffolds. In contrast to many conventional treatments that rely on animal or human-derived tissues, ROTIUM offers a 100% synthetic solution that fully resorbs within 3-4 months, with no reported adverse reactions in patients.
“Atreon Orthopedics is dedicated to advancing regenerative solutions that enable surgeons to deliver improved outcomes for patients while minimizing risks, costs, and procedural complexity,” said Ronald Bracken, CEO of Atreon Orthopedics. “With over 600,000 rotator cuff repairs performed annually in the U.S., and many of these procedures failing to achieve proper tendon-bone healing, ROTIUM addresses an urgent need for better biologic solutions in orthopedic surgery.”
Looking ahead, Atreon Orthopedics will expand its tissue remodeling platform to new tendon healing applications in the shoulder and beyond, including foot & ankle, knee and hip, launching new products in early 2025.
Source: Atreon Orthopedics
Atreon Orthopedics announced the milestone of the successful use of its ROTIUM Bioresorbable Wick in over 10,000 rotator cuff repair surgeries. The implant, which was granted initial FDA 510(k) clearance in 2019, is available in the U.S., Taiwan and other select countries worldwide.
The ROTIUM implant is a fully resorbable,...
Atreon Orthopedics announced the milestone of the successful use of its ROTIUM Bioresorbable Wick in over 10,000 rotator cuff repair surgeries. The implant, which was granted initial FDA 510(k) clearance in 2019, is available in the U.S., Taiwan and other select countries worldwide.
The ROTIUM implant is a fully resorbable, synthetic-nanofiber scaffold designed to optimize healing at the critical tendon/bone interface in rotator cuff repairs. By supporting the body’s natural healing processes and promoting better tissue integration, ROTIUM can help surgeons achieve stronger, more consistently durable repairs.
The technology’s rapid adoption in the market may be attributed to three key factors:
- Data-Driven Efficacy & Safety – Proven results that support positive patient outcomes.
- Cost-Effectiveness – Affordable without compromising quality, appealing to price-sensitive markets.
- Ease of Use – A simplified approach to implantation that enhances surgical efficiency.
The ROTIUM technology is the culmination of over 16 years of research in tissue remodeling, utilizing Electrospun engineered scaffolds. In contrast to many conventional treatments that rely on animal or human-derived tissues, ROTIUM offers a 100% synthetic solution that fully resorbs within 3-4 months, with no reported adverse reactions in patients.
“Atreon Orthopedics is dedicated to advancing regenerative solutions that enable surgeons to deliver improved outcomes for patients while minimizing risks, costs, and procedural complexity,” said Ronald Bracken, CEO of Atreon Orthopedics. “With over 600,000 rotator cuff repairs performed annually in the U.S., and many of these procedures failing to achieve proper tendon-bone healing, ROTIUM addresses an urgent need for better biologic solutions in orthopedic surgery.”
Looking ahead, Atreon Orthopedics will expand its tissue remodeling platform to new tendon healing applications in the shoulder and beyond, including foot & ankle, knee and hip, launching new products in early 2025.
Source: Atreon Orthopedics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







