
 Copy to clipboard
Copy to clipboard 
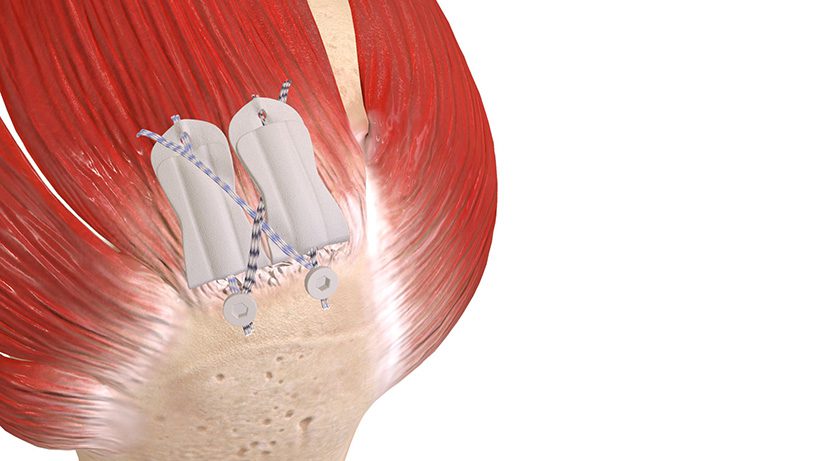
Atreon Orthopedics announced FDA 510(k) clearance and full U.S. launch of BioCharge Autobiologic Matrix, a bioresorbable synthetic implant designed to address biological failure modes in rotator cuff repair while improving repair integrity and long-term patient outcomes.
BioCharge is designed as an onlay scaffold to:
- Kickstart the patient’s natural healing response – wick active biology at the tendon’s bursal side of the rotator cuff repair
- Promote cellular activity to encourage native collagen remodeling – deter scar tissue formation
- Reinforce the suture-tendon interface – minimize suture cut-through failure risk
Atreon’s electrospun nanofiber platform technology is an alternative to traditional augment devices, which rely on animal-processed collagen or human dermal allografts that carry inherent patient compatibility risks and have a costly manufacturing process. With 17 years of tissue remodeling research, Atreon’s design mimics the natural structure of tissues and drives cellular attachment and proliferation more effectively than collagen-based products1. Additionally, the resorption process of the fibers poly(glycolic acid) and poly(lactide-co-caprolactone) serves a purpose—breaking down in a way that offers benefits to the healing cascade, including anti-inflammatory effects, enhanced vascular remodeling, and accelerated cellular migration1.
“We’re excited to expand our rotator cuff augmentation portfolio, building on the proven success of our flagship product, the ROTIUM® Bioresorbable Wick,” said Ronald Bracken, CEO of Atreon. “Retear rates remain high due to the complexities of tendon disease. With the introduction of BioCharge, Atreon is the only company offering solutions engineered to support native remodeling at both the tendon-bone and tendon-suture interfaces, resulting in improved treatment options for surgeons and their patients.”
Source: Atreon Orthopedics
Atreon Orthopedics announced FDA 510(k) clearance and full U.S. launch of BioCharge Autobiologic Matrix, a bioresorbable synthetic implant designed to address biological failure modes in rotator cuff repair while improving repair integrity and long-term patient outcomes.
BioCharge is designed as an onlay scaffold to:
Kickstart the...
Atreon Orthopedics announced FDA 510(k) clearance and full U.S. launch of BioCharge Autobiologic Matrix, a bioresorbable synthetic implant designed to address biological failure modes in rotator cuff repair while improving repair integrity and long-term patient outcomes.
BioCharge is designed as an onlay scaffold to:
- Kickstart the patient’s natural healing response – wick active biology at the tendon’s bursal side of the rotator cuff repair
- Promote cellular activity to encourage native collagen remodeling – deter scar tissue formation
- Reinforce the suture-tendon interface – minimize suture cut-through failure risk
Atreon’s electrospun nanofiber platform technology is an alternative to traditional augment devices, which rely on animal-processed collagen or human dermal allografts that carry inherent patient compatibility risks and have a costly manufacturing process. With 17 years of tissue remodeling research, Atreon’s design mimics the natural structure of tissues and drives cellular attachment and proliferation more effectively than collagen-based products1. Additionally, the resorption process of the fibers poly(glycolic acid) and poly(lactide-co-caprolactone) serves a purpose—breaking down in a way that offers benefits to the healing cascade, including anti-inflammatory effects, enhanced vascular remodeling, and accelerated cellular migration1.
“We’re excited to expand our rotator cuff augmentation portfolio, building on the proven success of our flagship product, the ROTIUM® Bioresorbable Wick,” said Ronald Bracken, CEO of Atreon. “Retear rates remain high due to the complexities of tendon disease. With the introduction of BioCharge, Atreon is the only company offering solutions engineered to support native remodeling at both the tendon-bone and tendon-suture interfaces, resulting in improved treatment options for surgeons and their patients.”
Source: Atreon Orthopedics

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







