
 Copy to clipboard
Copy to clipboard 
Atlas Spine announced FDA 510(k) clearance and imminent launch of the V3 Guided Segmental Plating System for anterior cervical discectomy and fusion (ACDF).
The V3 progressive technique combines attributes of both conventional plate/cage constructs and standalone interbody devices into one system and complements the HiJak™ AC Expandable Cervical Interbody Fusion Device which launched in 1Q19.
Compared to conventional plating, V3’s approach reduces the dissection and retraction required to perform an ACDF through use of smaller, less invasive, individual plates for multi-level constructs. This is designed to promote load sharing at each leve and avoid stress shielding that can result from single piece multi-level plates. When used with HiJAK, V3 can address each diseased segment individually. The guidance system eliminates the fiddle factor during installation by allowing the surgeon to temporarily dock the plate onto the face of the HiJAK device.
Compared to zero profile standalone devices, V3 offers increased rigidity by placing the plate on the anterior sideof the vertebral body. The plate allows an easier screw angulation, avoiding angled drivers and the risk of violating the endplate. Studies indicate that the segmental technique has shown greater fusion rates than standalone devices for both single and multi-level constructs.
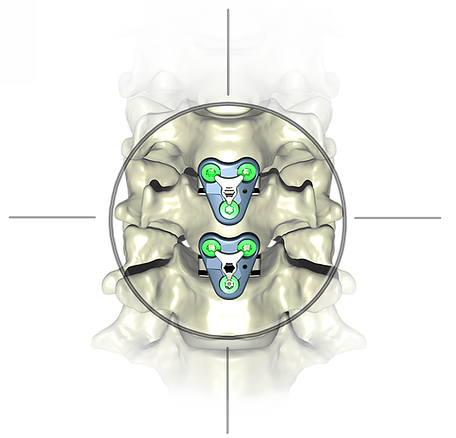
Source: Atlas Spine Inc.
Atlas Spine announced FDA 510(k) clearance and imminent launch of the V3 Guided Segmental Plating System for anterior cervical discectomy and fusion (ACDF).
The V3 progressive technique combines attributes of both conventional plate/cage constructs and standalone interbody devices into one system and complements the HiJak™ AC Expandable...
Atlas Spine announced FDA 510(k) clearance and imminent launch of the V3 Guided Segmental Plating System for anterior cervical discectomy and fusion (ACDF).
The V3 progressive technique combines attributes of both conventional plate/cage constructs and standalone interbody devices into one system and complements the HiJak™ AC Expandable Cervical Interbody Fusion Device which launched in 1Q19.
Compared to conventional plating, V3’s approach reduces the dissection and retraction required to perform an ACDF through use of smaller, less invasive, individual plates for multi-level constructs. This is designed to promote load sharing at each leve and avoid stress shielding that can result from single piece multi-level plates. When used with HiJAK, V3 can address each diseased segment individually. The guidance system eliminates the fiddle factor during installation by allowing the surgeon to temporarily dock the plate onto the face of the HiJAK device.
Compared to zero profile standalone devices, V3 offers increased rigidity by placing the plate on the anterior sideof the vertebral body. The plate allows an easier screw angulation, avoiding angled drivers and the risk of violating the endplate. Studies indicate that the segmental technique has shown greater fusion rates than standalone devices for both single and multi-level constructs.

Source: Atlas Spine Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







