
 Copy to clipboard
Copy to clipboard 
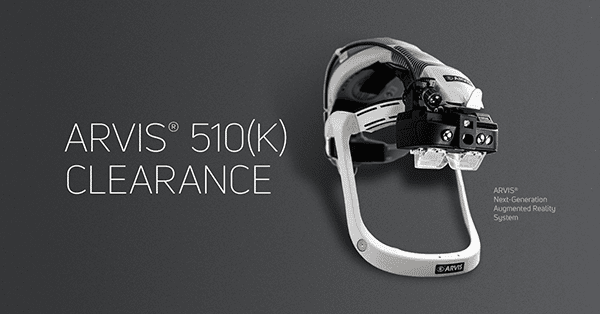
Insight Medical Systems gained FDA 510(k) clearance to market ARVIS® (Augmented Reality Visualization and Information System). This is the first wearable surgical navigation system to be cleared for both hip and knee arthroplasty. ARVIS combines tracking cameras with both a 3D display and handsfree interface in an integrated eyepiece for total joint arthroplasty. ARVIS is the first system with proprietary hardware designed to assist arthroplasty surgeons in enhancing component positioning precision to improve joint arthroplasty outcomes.
“This is an important milestone for Insight. We are excited to move forward with commercializing ARVIS with our partner DJO and to bring the benefits of augmented reality to hip and knee surgeons without the typical cost and complexity,” said Nick van der Walt, CEO of Insight.
In March 2021, DJO® announced a strategic investment in Insight Medical Systems to support DJO’s commitment to the growing computer-assisted surgery market. ARVIS complements DJO’s ASC 360™ solutions and adds to their range of implant solutions, surgical tools and digital care solutions that help position DJO as a leader in the rapidly expanding ASC setting.
Source: Insight Medical Systems
Insight Medical Systems gained FDA 510(k) clearance to market ARVIS® (Augmented Reality Visualization and Information System). This is the first wearable surgical navigation system to be cleared for both hip and knee arthroplasty. ARVIS combines tracking cameras with both a 3D display and handsfree interface in an integrated eyepiece for total...
Insight Medical Systems gained FDA 510(k) clearance to market ARVIS® (Augmented Reality Visualization and Information System). This is the first wearable surgical navigation system to be cleared for both hip and knee arthroplasty. ARVIS combines tracking cameras with both a 3D display and handsfree interface in an integrated eyepiece for total joint arthroplasty. ARVIS is the first system with proprietary hardware designed to assist arthroplasty surgeons in enhancing component positioning precision to improve joint arthroplasty outcomes.
“This is an important milestone for Insight. We are excited to move forward with commercializing ARVIS with our partner DJO and to bring the benefits of augmented reality to hip and knee surgeons without the typical cost and complexity,” said Nick van der Walt, CEO of Insight.
In March 2021, DJO® announced a strategic investment in Insight Medical Systems to support DJO’s commitment to the growing computer-assisted surgery market. ARVIS complements DJO’s ASC 360™ solutions and adds to their range of implant solutions, surgical tools and digital care solutions that help position DJO as a leader in the rapidly expanding ASC setting.
Source: Insight Medical Systems

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.






