
 Copy to clipboard
Copy to clipboard 
Articulus Bio received an undisclosed amount of funding from AngelMD. The company is developing a total ankle replacement specifically addressing the issue of wear debris.
Articulus seeks capital to collect performance data on sub-assembly components of its Tendonoid™ Web Joint technology, which is designed to reduce and eliminate friction at articulating joint faces.
Tendonoid technology is based on a novel configuration of Ultra High Molecular Weight Polyethylene (UHMWPE), used to create an implant that “floats” the patient’s weight on a web of UHMWPE cords to allow anatomical range of motion without wear debris generation. The total ankle device and custom jig are 3D-printed from CT scan data for just-in-time, per-patient use. Articulus intends to take lessons learned from its total ankle device and investigate application in other large and small joints.
Funds from AngelMD will also support pursuit of a Q-Submission meeting with FDA and the continued build of Articulus’ intellectual property portfolio.
Sources: AngelMD; AngelMD Blog; Articulus Bio profile on AngelMD
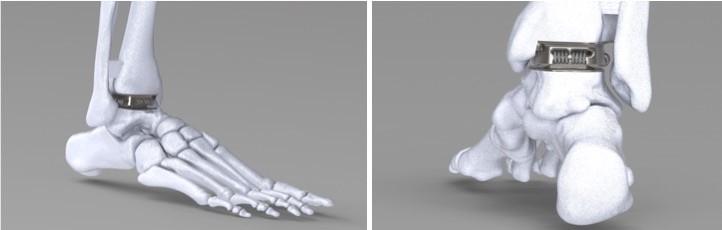
Image retrieved from the AngelMD Blog
Articulus Bio received an undisclosed amount of funding from AngelMD. The company is developing a total ankle replacement specifically addressing the issue of wear debris.
Articulus seeks capital to collect performance data on sub-assembly components of its Tendonoid™ Web Joint technology, which is designed to reduce and eliminate friction...
Articulus Bio received an undisclosed amount of funding from AngelMD. The company is developing a total ankle replacement specifically addressing the issue of wear debris.
Articulus seeks capital to collect performance data on sub-assembly components of its Tendonoid™ Web Joint technology, which is designed to reduce and eliminate friction at articulating joint faces.
Tendonoid technology is based on a novel configuration of Ultra High Molecular Weight Polyethylene (UHMWPE), used to create an implant that “floats” the patient’s weight on a web of UHMWPE cords to allow anatomical range of motion without wear debris generation. The total ankle device and custom jig are 3D-printed from CT scan data for just-in-time, per-patient use. Articulus intends to take lessons learned from its total ankle device and investigate application in other large and small joints.
Funds from AngelMD will also support pursuit of a Q-Submission meeting with FDA and the continued build of Articulus’ intellectual property portfolio.
Sources: AngelMD; AngelMD Blog; Articulus Bio profile on AngelMD

Image retrieved from the AngelMD Blog

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







