
 Copy to clipboard
Copy to clipboard 
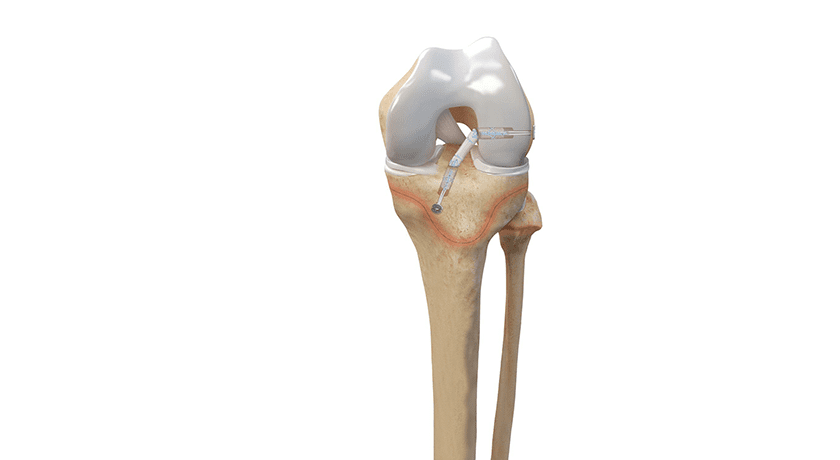
Arthrex received FDA 510(k) clearance to market the ACL TightRope implant for pediatric indications. The device is reportedly the first and only fixation device for anterior cruciate ligament (ACL) injuries cleared for pediatric use.
Arthrex developed all-epiphyseal and transphyseal techniques and instrumentation for ACL surgery alongside orthopedic surgeons from the Hospital for Special Surgery.
The Arthrex all-epiphyseal technique was developed for skeletally immature patients and involves avoiding the pediatric growth plates to repair or reconstruct the ACL. With the pediatric-specific instrumentation guides, surgeons drill sockets for the new, reconstructed ACL by avoiding the growth plate to diminish the potential for growth disturbance.
In older adolescent patients who are approaching skeletal maturation, surgeons will drill across the growth plates to reconstruct the ACL using an all-soft-tissue autograft. Of the two all-soft-tissue autografts available for use in this young athlete population, quadriceps autografts have been found to have superior outcomes compared to hamstring autografts with lower revision rates and greater return-to-sport rates.
The Arthrex ACL TightRope portfolio includes the ACL TightRope II implant, the TightRope attachable button system and implant, the FiberTag® TightRope implant and the ACL Repair TightRope implant with FiberRing™ sutures.
Source: Arthrex
Arthrex received FDA 510(k) clearance to market the ACL TightRope implant for pediatric indications. The device is reportedly the first and only fixation device for anterior cruciate ligament (ACL) injuries cleared for pediatric use.
Arthrex developed all-epiphyseal and transphyseal techniques and instrumentation for ACL surgery alongside...
Arthrex received FDA 510(k) clearance to market the ACL TightRope implant for pediatric indications. The device is reportedly the first and only fixation device for anterior cruciate ligament (ACL) injuries cleared for pediatric use.
Arthrex developed all-epiphyseal and transphyseal techniques and instrumentation for ACL surgery alongside orthopedic surgeons from the Hospital for Special Surgery.
The Arthrex all-epiphyseal technique was developed for skeletally immature patients and involves avoiding the pediatric growth plates to repair or reconstruct the ACL. With the pediatric-specific instrumentation guides, surgeons drill sockets for the new, reconstructed ACL by avoiding the growth plate to diminish the potential for growth disturbance.
In older adolescent patients who are approaching skeletal maturation, surgeons will drill across the growth plates to reconstruct the ACL using an all-soft-tissue autograft. Of the two all-soft-tissue autografts available for use in this young athlete population, quadriceps autografts have been found to have superior outcomes compared to hamstring autografts with lower revision rates and greater return-to-sport rates.
The Arthrex ACL TightRope portfolio includes the ACL TightRope II implant, the TightRope attachable button system and implant, the FiberTag® TightRope implant and the ACL Repair TightRope implant with FiberRing™ sutures.
Source: Arthrex

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







