
 Copy to clipboard
Copy to clipboard 
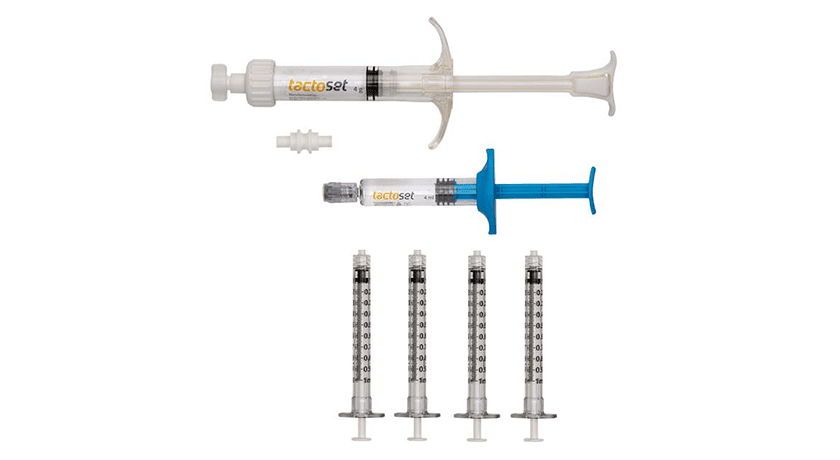
Anika Therapeutics received an additional FDA 510(k) clearance for Tactoset Injectable Bone Substitute. This new indication expands the use of Tactoset to be combined with autologous bone marrow aspirate (BMA), a source of various cellular and molecular components which have demonstrated positive effects on tissue regeneration in musculoskeletal injuries. This increases Tactoset’s commercial reach by combining BMA with Tactoset for the treatment of bone defects such as osteoporotic bone, cysts and insufficiency fractures.
Tactoset injectable, settable, calcium phosphate-based bone graft substitute incorporates Anika’s hyaluronic acid technology which makes the product highly flowable, easily injectable and able to interdigitate into trabecular bone architecture with improved handling characteristics compared to competitive products. Once injected, Tactoset hardens and mimics the properties of normal trabecular bone initially before remodeling into healthy bone over time.
Further, Anika has signed an agreement to distribute its Marrow Cellution Bone Marrow Aspiration Needle in the U.S. This system combines aspiration and cannula motion to maximize cell recovery, while eliminating the need to remove materials from the sterile field for centrifuge processing. Finally, Anika also recently launched enhanced Tactoset delivery cannulas, designed to improve ease of use and simplify the targeting and delivery of the material, especially in foot and ankle procedures.
Cheryl R. Blanchard, PhD, President and CEO, Anika Therapeutics, said, “This new FDA clearance for mixing Tactoset with BMA provides a product with improved regenerative capacity and showcases Anika’s commitment to developing unique solutions that are meaningful to our customers and their patients. It also further reinforces our confidence that we can increase Tactoset’s addressable market to well beyond $100 million by creating a new market for hardware augmentation.”
Source: Anika
Anika Therapeutics received an additional FDA 510(k) clearance for Tactoset Injectable Bone Substitute. This new indication expands the use of Tactoset to be combined with autologous bone marrow aspirate (BMA), a source of various cellular and molecular components which have demonstrated positive effects on tissue regeneration in musculoskeletal...
Anika Therapeutics received an additional FDA 510(k) clearance for Tactoset Injectable Bone Substitute. This new indication expands the use of Tactoset to be combined with autologous bone marrow aspirate (BMA), a source of various cellular and molecular components which have demonstrated positive effects on tissue regeneration in musculoskeletal injuries. This increases Tactoset’s commercial reach by combining BMA with Tactoset for the treatment of bone defects such as osteoporotic bone, cysts and insufficiency fractures.
Tactoset injectable, settable, calcium phosphate-based bone graft substitute incorporates Anika’s hyaluronic acid technology which makes the product highly flowable, easily injectable and able to interdigitate into trabecular bone architecture with improved handling characteristics compared to competitive products. Once injected, Tactoset hardens and mimics the properties of normal trabecular bone initially before remodeling into healthy bone over time.
Further, Anika has signed an agreement to distribute its Marrow Cellution Bone Marrow Aspiration Needle in the U.S. This system combines aspiration and cannula motion to maximize cell recovery, while eliminating the need to remove materials from the sterile field for centrifuge processing. Finally, Anika also recently launched enhanced Tactoset delivery cannulas, designed to improve ease of use and simplify the targeting and delivery of the material, especially in foot and ankle procedures.
Cheryl R. Blanchard, PhD, President and CEO, Anika Therapeutics, said, “This new FDA clearance for mixing Tactoset with BMA provides a product with improved regenerative capacity and showcases Anika’s commitment to developing unique solutions that are meaningful to our customers and their patients. It also further reinforces our confidence that we can increase Tactoset’s addressable market to well beyond $100 million by creating a new market for hardware augmentation.”
Source: Anika

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







