Copy to clipboard
Copy to clipboard 
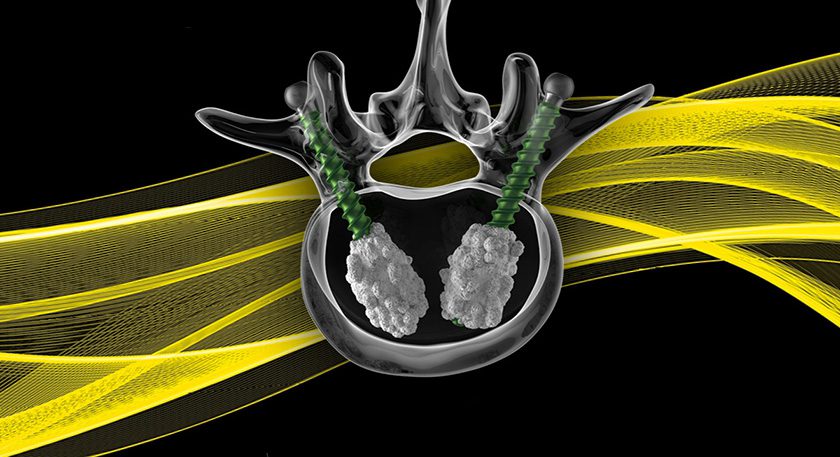
Anika Therapeutics completed enrollment of 576 patients in its second pivotal Phase III trial evaluating CINGAL® hyaluronic acid/corticosteroid viscosupplement for the treatment of knee osteoarthritis symptoms.
CINGAL combines triamcinolone hexacetonide, an FDA-cleared steroid utilized to treat inflammation, with cross-linked, non-animal-derived hyaluronic acid. The trial will assess safety and efficacy of CINGAL in improvements to pain, function and quality of life over 26 weeks vs. MONOVISC® plus the same steriod used in CINGAL.
Results from the initial Phase III trial demonstrated superior short term pain relief after CINGAL injection vs. HA alone, and superior relief from OA-related pain, stiffness and function through 26 weeks vs. saline.
Anika expects to complete this second pivotal Phase III trial in 1H18 and obtain FDA approval the following year.
Sources: Anika Therapeutics, Inc.; ORTHOWORLD Inc.
Anika Therapeutics completed enrollment of 576 patients in its second pivotal Phase III trial evaluating CINGAL® hyaluronic acid/corticosteroid viscosupplement for the treatment of knee osteoarthritis symptoms.
CINGAL combines triamcinolone hexacetonide, an FDA-cleared steroid utilized to treat inflammation, with cross-linked,...
Anika Therapeutics completed enrollment of 576 patients in its second pivotal Phase III trial evaluating CINGAL® hyaluronic acid/corticosteroid viscosupplement for the treatment of knee osteoarthritis symptoms.
CINGAL combines triamcinolone hexacetonide, an FDA-cleared steroid utilized to treat inflammation, with cross-linked, non-animal-derived hyaluronic acid. The trial will assess safety and efficacy of CINGAL in improvements to pain, function and quality of life over 26 weeks vs. MONOVISC® plus the same steriod used in CINGAL.
Results from the initial Phase III trial demonstrated superior short term pain relief after CINGAL injection vs. HA alone, and superior relief from OA-related pain, stiffness and function through 26 weeks vs. saline.
Anika expects to complete this second pivotal Phase III trial in 1H18 and obtain FDA approval the following year.
Sources: Anika Therapeutics, Inc.; ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.