
 Copy to clipboard
Copy to clipboard 
Amphix Bio received a Breakthrough Device Designation from FDA for a drug/device combination product for bone regeneration. The designation covers the use of the therapeutic device to treat degenerative disc disease with transforaminal lumbar interbody fusion procedures.
This is the first product based on supramolecular peptide amphiphiles — Amphix Bio’s core technology platform — to be evaluated by FDA.
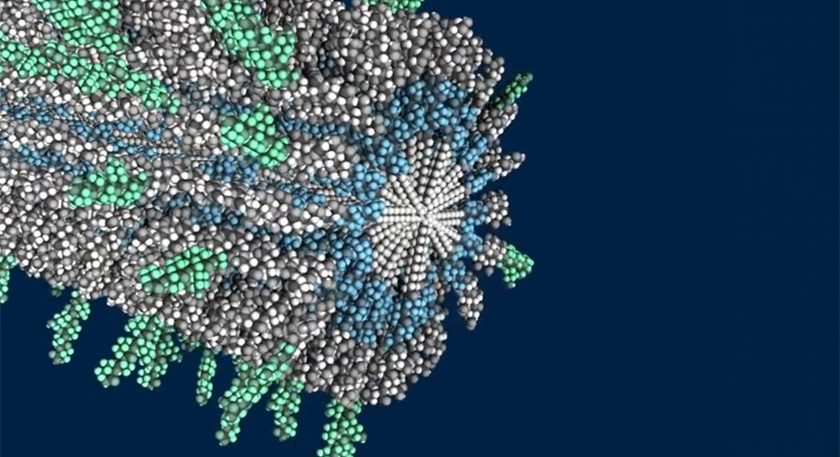
Amphix Bio is developing a new class of supramolecular therapies that instruct cells to initiate regenerative processes and restore function lost from injury, disease or aging. These therapies contain tens of thousands of biological signals that trigger targeted signaling pathways while forming scaffolds to support tissue growth. The company states that its technology enables effective regeneration of cells and tissues without the manufacturing challenges and safety concerns of approaches like cell therapies. Its research pipeline includes bone, neural and cartilage regeneration programs.
“This designation from the FDA is a major milestone for supramolecular therapeutics, and validates the high unmet need that our approach addresses,” said Samuel Stupp, PhD, co-founder and Chief Scientific Officer of Amphix Bio. “The expedited assessment and review are especially important given that we are aiming to advance an entirely new regenerative medicine platform to the clinic.”
Source: Amphix Bio
Amphix Bio received a Breakthrough Device Designation from FDA for a drug/device combination product for bone regeneration. The designation covers the use of the therapeutic device to treat degenerative disc disease with transforaminal lumbar interbody fusion procedures.
This is the first product based on supramolecular peptide amphiphiles —...
Amphix Bio received a Breakthrough Device Designation from FDA for a drug/device combination product for bone regeneration. The designation covers the use of the therapeutic device to treat degenerative disc disease with transforaminal lumbar interbody fusion procedures.
This is the first product based on supramolecular peptide amphiphiles — Amphix Bio’s core technology platform — to be evaluated by FDA.
Amphix Bio is developing a new class of supramolecular therapies that instruct cells to initiate regenerative processes and restore function lost from injury, disease or aging. These therapies contain tens of thousands of biological signals that trigger targeted signaling pathways while forming scaffolds to support tissue growth. The company states that its technology enables effective regeneration of cells and tissues without the manufacturing challenges and safety concerns of approaches like cell therapies. Its research pipeline includes bone, neural and cartilage regeneration programs.
“This designation from the FDA is a major milestone for supramolecular therapeutics, and validates the high unmet need that our approach addresses,” said Samuel Stupp, PhD, co-founder and Chief Scientific Officer of Amphix Bio. “The expedited assessment and review are especially important given that we are aiming to advance an entirely new regenerative medicine platform to the clinic.”
Source: Amphix Bio

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







