Copy to clipboard
Copy to clipboard 
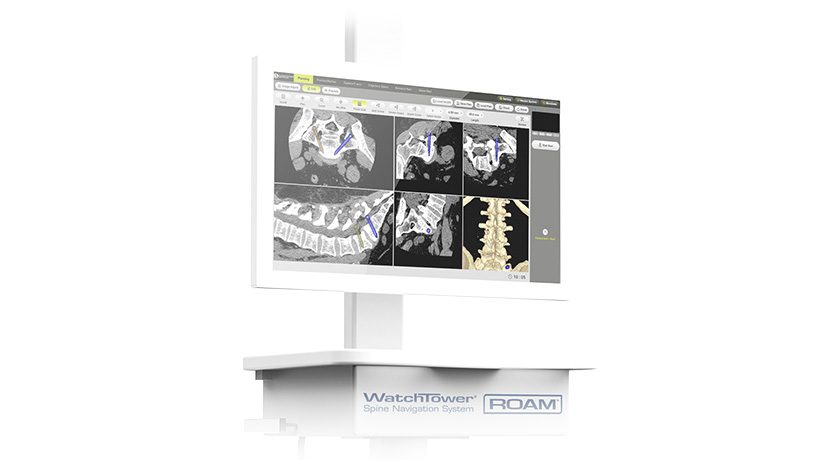
Amedica (AMDA) submitted its responses to FDA in relation to the CASCADE clinical trial.
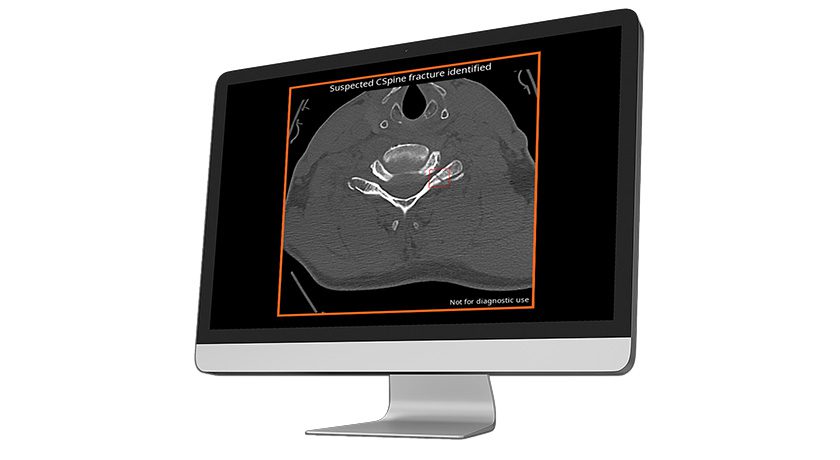
CASCADE compared 24-month outcomes from single-level cervical fusion between AMDA’s porous silicon nitride vs. autograft bone. Data indicated that porous silicon nitride achieved clinical and radiographic outcomes that were comparable to autograft.
AMDA expects a final determination from FDA within 60 days.
Source: Amedica Corporation
Amedica (AMDA) submitted its responses to FDA in relation to the CASCADE clinical trial.
CASCADE compared 24-month outcomes from single-level cervical fusion between AMDA's porous silicon nitride vs. autograft bone. Data indicated that porous silicon nitride achieved clinical and radiographic outcomes that were...
Amedica (AMDA) submitted its responses to FDA in relation to the CASCADE clinical trial.
CASCADE compared 24-month outcomes from single-level cervical fusion between AMDA’s porous silicon nitride vs. autograft bone. Data indicated that porous silicon nitride achieved clinical and radiographic outcomes that were comparable to autograft.
AMDA expects a final determination from FDA within 60 days.
Source: Amedica Corporation

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.