
 Copy to clipboard
Copy to clipboard 
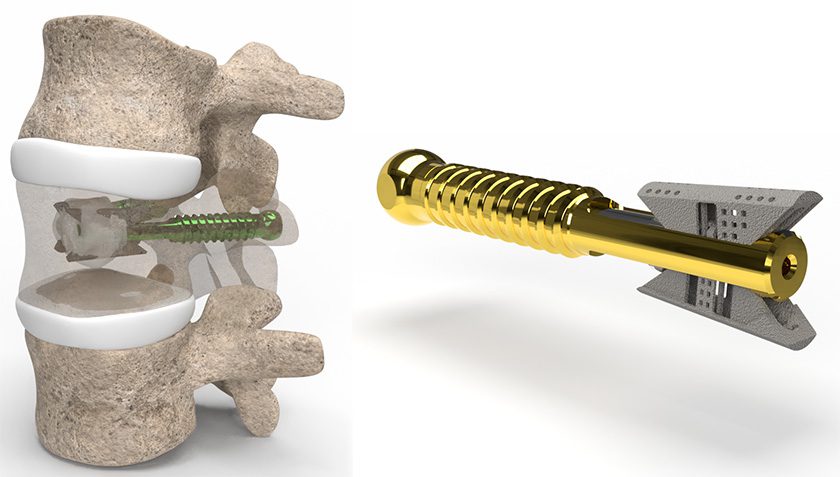
Amber Implants received FDA 510(k) clearance to market the VCFix Spinal System.
VCFix is a vertebral augmentation system designed to treat a broad spectrum of vertebral compression fractures. This minimally invasive solution is used with bone cement and provides strong anterior and posterior column support. It integrates seamlessly into existing surgical workflows, with the potential to improve spinal stabilization, reduce patient risk, and shorten procedure times, compared to current options.
One-year follow-up data from initial clinical trials of the system demonstrated immediate and sustained reduction in pain, improved spinal stability, and faster recovery. Amber Implants is also working towards CE Marking in the EU, aiming for broad labeling that supports both stand-alone use of VCFix and integration with posterior fixation systems.
Dr. Mohammad Ahmadi, Co-Founder and Chief Technology Officer of Amber Implants, said, “We will begin our US commercial launch with a pilot program in early 2026, accompanied by the expansion of the EXPAND pivotal trial into the US. This will be followed by broader physician availability from later in 2026. At the same time, we continue to pursue EU label expansion for stand-alone use and integration with one level fixation.”
Source: Amber Implants
Amber Implants received FDA 510(k) clearance to market the VCFix Spinal System.
VCFix is a vertebral augmentation system designed to treat a broad spectrum of vertebral compression fractures. This minimally invasive solution is used with bone cement and provides strong anterior and posterior column support. It integrates seamlessly into...
Amber Implants received FDA 510(k) clearance to market the VCFix Spinal System.
VCFix is a vertebral augmentation system designed to treat a broad spectrum of vertebral compression fractures. This minimally invasive solution is used with bone cement and provides strong anterior and posterior column support. It integrates seamlessly into existing surgical workflows, with the potential to improve spinal stabilization, reduce patient risk, and shorten procedure times, compared to current options.
One-year follow-up data from initial clinical trials of the system demonstrated immediate and sustained reduction in pain, improved spinal stability, and faster recovery. Amber Implants is also working towards CE Marking in the EU, aiming for broad labeling that supports both stand-alone use of VCFix and integration with posterior fixation systems.
Dr. Mohammad Ahmadi, Co-Founder and Chief Technology Officer of Amber Implants, said, “We will begin our US commercial launch with a pilot program in early 2026, accompanied by the expansion of the EXPAND pivotal trial into the US. This will be followed by broader physician availability from later in 2026. At the same time, we continue to pursue EU label expansion for stand-alone use and integration with one level fixation.”
Source: Amber Implants

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







