
 Copy to clipboard
Copy to clipboard 
Altior Trauma received FDA 510(k) clearance to market the Artemis Proximal Femoral Nail. Marketing and sales will commence within 1Q21.
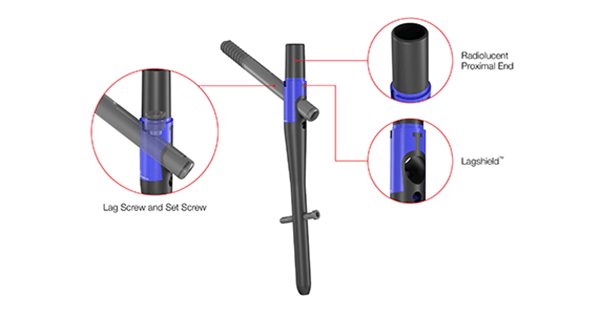
Artemis combines titanium with a blend of Solvay Zeniva® carbon fiber-reinforced polyetheretherketone polymer. The combined materials support skeletal visualization and promotion of fracture healing.
The Artemis System provides a fourth-generation nail with surgical instruments that allow for simplified implantation. The instrument set includes a novel anti-rotation pin that is designed to prevent head and neck rotation during reaming and implantation of the lag screw.
“The Artemis System is truly a global product platform that delivers modern innovation. I am incredibly proud of our engineers and what they have accomplished in order to deliver a next generation hip fracture nail utilizing a patent pending advanced manufacturing process that significantly reduces costs,” said Vadim Gurevich, CEO of Altior Trauma. “The launch of Artemis, our first of many products, will announce to the world that we are here to improve hip fracture technology and care, while reducing the overall cost to the healthcare system.”
Altior Trauma received FDA 510(k) clearance to market the Artemis Proximal Femoral Nail. Marketing and sales will commence within 1Q21.
Artemis combines titanium with a blend of Solvay Zeniva® carbon fiber-reinforced polyetheretherketone polymer. The combined materials support skeletal visualization and promotion of fracture healing.
...
Altior Trauma received FDA 510(k) clearance to market the Artemis Proximal Femoral Nail. Marketing and sales will commence within 1Q21.
Artemis combines titanium with a blend of Solvay Zeniva® carbon fiber-reinforced polyetheretherketone polymer. The combined materials support skeletal visualization and promotion of fracture healing.
The Artemis System provides a fourth-generation nail with surgical instruments that allow for simplified implantation. The instrument set includes a novel anti-rotation pin that is designed to prevent head and neck rotation during reaming and implantation of the lag screw.
“The Artemis System is truly a global product platform that delivers modern innovation. I am incredibly proud of our engineers and what they have accomplished in order to deliver a next generation hip fracture nail utilizing a patent pending advanced manufacturing process that significantly reduces costs,” said Vadim Gurevich, CEO of Altior Trauma. “The launch of Artemis, our first of many products, will announce to the world that we are here to improve hip fracture technology and care, while reducing the overall cost to the healthcare system.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







