
 Copy to clipboard
Copy to clipboard 
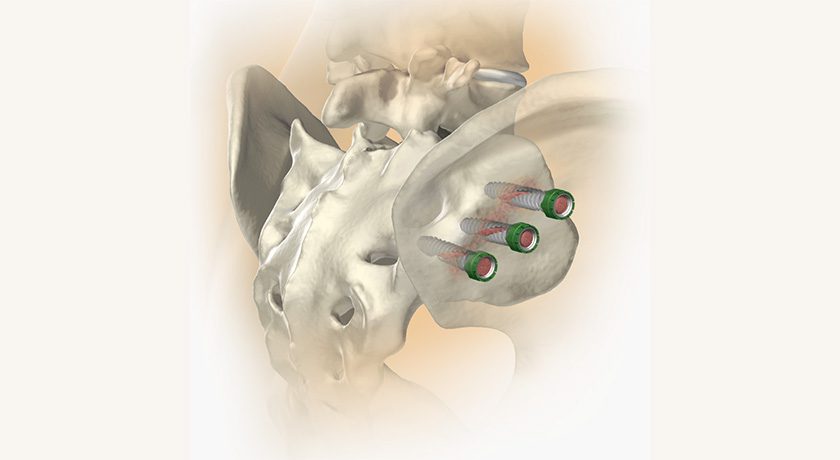
Alevio Spine announced FDA 510(k) clearance of additional options for the SI-Cure Implant family. The clearance adds headless screws and a range of additional screw size options to the previously cleared system.
The SI-Cure Foundation construct provides an option for patients who are undergoing sacropelvic fixation as part of a lumbar or thoracolumbar fusion. A Retrospective Cohort Study using SI-Cure Implants reported that simultaneous SIJ instrumentation and fusion decrease the risk of disability, prevent the development of postoperative SIJ pain and may also protect the S2AI screw from loosening and failure.
SI-Cure Implant System Highlights:
- Patented design provides graft contact along the length of the Implant
- Features a Self-Harvesting design that collects autograft as the Implant is advanced, thus eliminating the need for bone graft
- As the Implant is advanced, the helical design pushes the bone into the open architecture of the implant allowing the Self-Advancing autograft to backfill the length of the implant
The SI-Cure Sacroiliac Joint Fusion System consists of titanium implants of 7mm, 8.5mm, 9.5mm, 10.5mm and 11mm diameter with lengths from 35-110mm to accommodate patient anatomy. An optional serrated washer can be placed on the screw head for load distribution.
Source: Alevio Spine
Alevio Spine announced FDA 510(k) clearance of additional options for the SI-Cure Implant family. The clearance adds headless screws and a range of additional screw size options to the previously cleared system.
The SI-Cure Foundation construct provides an option for patients who are undergoing sacropelvic fixation as part of a lumbar or...
Alevio Spine announced FDA 510(k) clearance of additional options for the SI-Cure Implant family. The clearance adds headless screws and a range of additional screw size options to the previously cleared system.
The SI-Cure Foundation construct provides an option for patients who are undergoing sacropelvic fixation as part of a lumbar or thoracolumbar fusion. A Retrospective Cohort Study using SI-Cure Implants reported that simultaneous SIJ instrumentation and fusion decrease the risk of disability, prevent the development of postoperative SIJ pain and may also protect the S2AI screw from loosening and failure.
SI-Cure Implant System Highlights:
- Patented design provides graft contact along the length of the Implant
- Features a Self-Harvesting design that collects autograft as the Implant is advanced, thus eliminating the need for bone graft
- As the Implant is advanced, the helical design pushes the bone into the open architecture of the implant allowing the Self-Advancing autograft to backfill the length of the implant
The SI-Cure Sacroiliac Joint Fusion System consists of titanium implants of 7mm, 8.5mm, 9.5mm, 10.5mm and 11mm diameter with lengths from 35-110mm to accommodate patient anatomy. An optional serrated washer can be placed on the screw head for load distribution.
Source: Alevio Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







