
 Copy to clipboard
Copy to clipboard 
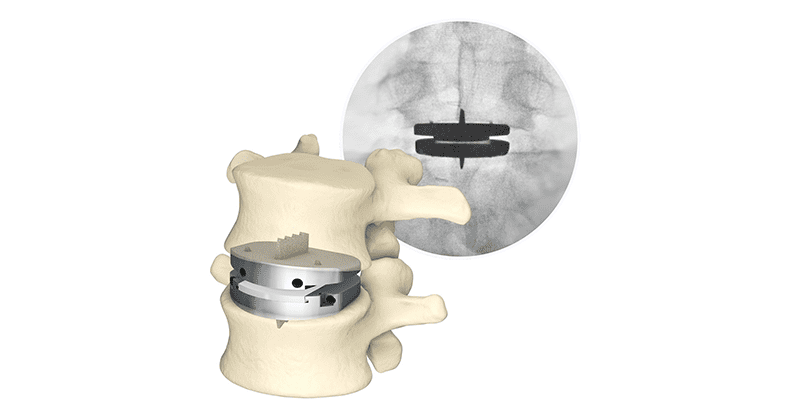
Centinel Spine announced significant expansion to patient access for the prodisc L Lumbar Total Disc Replacement (TDR). An additional 22 million lives will now be covered as the result of an update to medical necessity guidelines by Aetna to include a positive coverage policy recommendation for one-level lumbar TDR.
Aetna is one of the largest commercial insurers in the U.S. and previously had a coverage policy that considered one-level lumbar TDR “experimental and investigational,” and thus not covered. The new positive coverage policy opens access to patients with one-level symptomatic degenerative disc disease (DDD) who may benefit from total disc replacement using a technology such as prodisc L. With Aetna’s coverage update, all major commercial payors now cover one-level lumbar TDR.
Further, effective January 1, 2023, providers began to utilize a new Current Procedural Terminology (CPT) code when performing two-level lumbar total disc arthroplasty via an anterior approach. Centinel Spine’s prodisc L is the only FDA-approved TDR system in the U.S. for two-level use in the lumbar spine. The company has also recently launched prodisc L Anatomic Endplates, designed to shift the lordotic angle of the lumbar implant to the inferior endplate.
Source: Centinel Spine, LLC
Centinel Spine announced significant expansion to patient access for the prodisc L Lumbar Total Disc Replacement (TDR). An additional 22 million lives will now be covered as the result of an update to medical necessity guidelines by Aetna to include a positive coverage policy recommendation for one-level lumbar TDR.
Aetna is one of the largest...
Centinel Spine announced significant expansion to patient access for the prodisc L Lumbar Total Disc Replacement (TDR). An additional 22 million lives will now be covered as the result of an update to medical necessity guidelines by Aetna to include a positive coverage policy recommendation for one-level lumbar TDR.
Aetna is one of the largest commercial insurers in the U.S. and previously had a coverage policy that considered one-level lumbar TDR “experimental and investigational,” and thus not covered. The new positive coverage policy opens access to patients with one-level symptomatic degenerative disc disease (DDD) who may benefit from total disc replacement using a technology such as prodisc L. With Aetna’s coverage update, all major commercial payors now cover one-level lumbar TDR.
Further, effective January 1, 2023, providers began to utilize a new Current Procedural Terminology (CPT) code when performing two-level lumbar total disc arthroplasty via an anterior approach. Centinel Spine’s prodisc L is the only FDA-approved TDR system in the U.S. for two-level use in the lumbar spine. The company has also recently launched prodisc L Anatomic Endplates, designed to shift the lordotic angle of the lumbar implant to the inferior endplate.
Source: Centinel Spine, LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







