Copy to clipboard
Copy to clipboard 
Aegis Spine, subsidiary of L&K BioMed, was granted FDA 510(k) clearance for AccelFix Expandable Cages. The devices launched in the U.S. earlier this month.
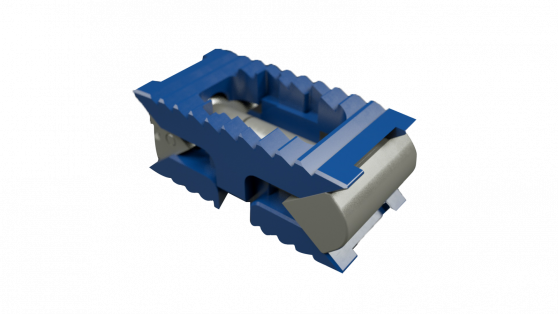
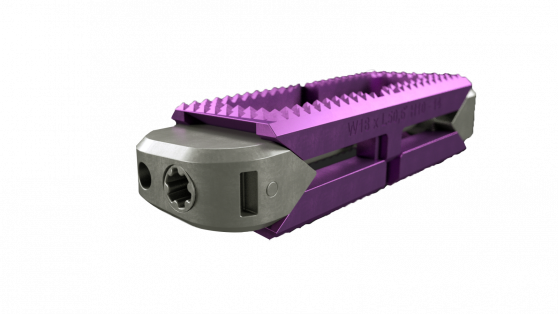
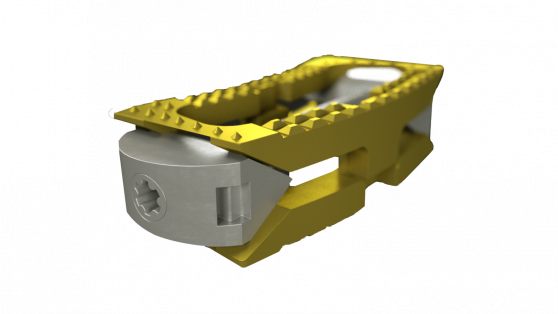
The system, pictured below, comprises sterile-packaged titanium alloy-based TLIF/PLIF cages (AccelFix-XT), an expandable lateral interbody (AccelFix-XL), and an Anterior-to-Psoas expandable interbody specifically contoured to avoid the contralateral nerve root (Accelfix-XTP).
AccelFix devices are complemented by a 5.5 rod spinal fixation system which earned FDA 510(k) clearance earlier this year.
Source: Aegis Spine, Inc.
Aegis Spine, subsidiary of L&K BioMed, was granted FDA 510(k) clearance for AccelFix Expandable Cages. The devices launched in the U.S. earlier this month.
The system, pictured below, comprises sterile-packaged titanium alloy-based TLIF/PLIF cages (AccelFix-XT), an expandable lateral interbody (AccelFix-XL), and an Anterior-to-Psoas...
Aegis Spine, subsidiary of L&K BioMed, was granted FDA 510(k) clearance for AccelFix Expandable Cages. The devices launched in the U.S. earlier this month.
The system, pictured below, comprises sterile-packaged titanium alloy-based TLIF/PLIF cages (AccelFix-XT), an expandable lateral interbody (AccelFix-XL), and an Anterior-to-Psoas expandable interbody specifically contoured to avoid the contralateral nerve root (Accelfix-XTP).
AccelFix devices are complemented by a 5.5 rod spinal fixation system which earned FDA 510(k) clearance earlier this year.
Source: Aegis Spine, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.