
 Copy to clipboard
Copy to clipboard 
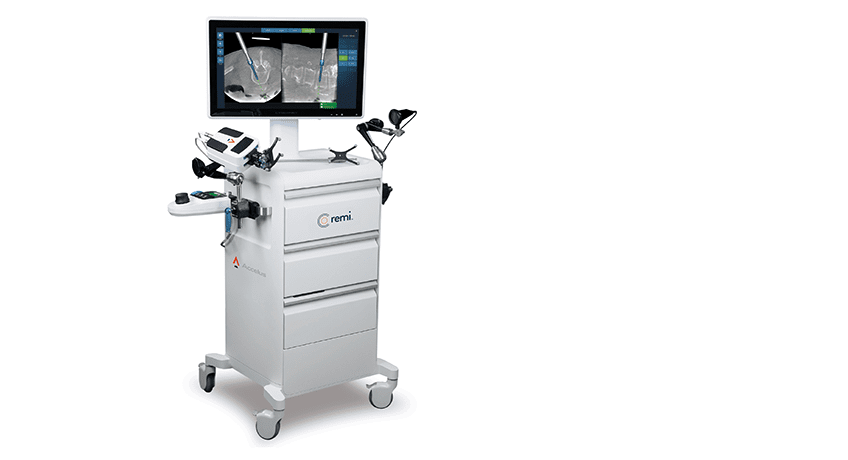
Accelus was granted FDA 510(k) clearance to market its Remi™ Robotic Navigation System software update that allows image capture with the GE OEC 3D, Ziehm Vision RFD 3D and Stryker Airo TruCT systems.
All Remi Robotic Navigation Systems in the field will be updated to the newly cleared software and will also continue to be compatible for use with O-arm imaging.
Remi is a targeting and navigation platform that provides robotic-assisted pedicle screw placement for surgeons performing lumbar spine fixation. It was cleared by FDA in 2021 with patient anatomy and fiducials identified through an O-arm scan. The new FDA clearance allows the system to be used with a broader portfolio of 3D imaging systems.
Remi was designed to address the limitations of legacy robotic systems in spine, including extended setup and teardown time, high costs, procedural workflow disruptions and taking up a large footprint in the OR.
“Cost, footprint and complexity were previously unscalable barriers that kept robotic spine surgery from being performed anywhere but at large hospitals; however, the Remi system’s new clearance provides smaller facilities and ASCs with access to its disruptive technology that can help precisely navigate for accurate screw placement with reduced reliance on traditional fluoroscopy,” said Chris Walsh, Accelus’s Chief Executive Officer and Co-Founder.
Source: Accelus
Accelus was granted FDA 510(k) clearance to market its Remi™ Robotic Navigation System software update that allows image capture with the GE OEC 3D, Ziehm Vision RFD 3D and Stryker Airo TruCT systems.
All Remi Robotic Navigation Systems in the field will be updated to the newly cleared software and will also continue to be compatible for use...
Accelus was granted FDA 510(k) clearance to market its Remi™ Robotic Navigation System software update that allows image capture with the GE OEC 3D, Ziehm Vision RFD 3D and Stryker Airo TruCT systems.
All Remi Robotic Navigation Systems in the field will be updated to the newly cleared software and will also continue to be compatible for use with O-arm imaging.
Remi is a targeting and navigation platform that provides robotic-assisted pedicle screw placement for surgeons performing lumbar spine fixation. It was cleared by FDA in 2021 with patient anatomy and fiducials identified through an O-arm scan. The new FDA clearance allows the system to be used with a broader portfolio of 3D imaging systems.
Remi was designed to address the limitations of legacy robotic systems in spine, including extended setup and teardown time, high costs, procedural workflow disruptions and taking up a large footprint in the OR.
“Cost, footprint and complexity were previously unscalable barriers that kept robotic spine surgery from being performed anywhere but at large hospitals; however, the Remi system’s new clearance provides smaller facilities and ASCs with access to its disruptive technology that can help precisely navigate for accurate screw placement with reduced reliance on traditional fluoroscopy,” said Chris Walsh, Accelus’s Chief Executive Officer and Co-Founder.
Source: Accelus

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







