
 Copy to clipboard
Copy to clipboard 

Active Implants NUsurface – Active Implants
Active Implants announced that two patients in Israel have undergone knee surgery with the company’s NUsurface® Meniscus Implant, the first artificial meniscus to be marketed in the Middle East. Previously, the device was only available in Israel for clinical trials.
Studies have shown that many meniscectomy patients continue to experience pain that affects the quality of life and can lead to knee replacement. Transplant tissue is scarce, and waiting lists exist in many countries. NUsurface offers an alternative.
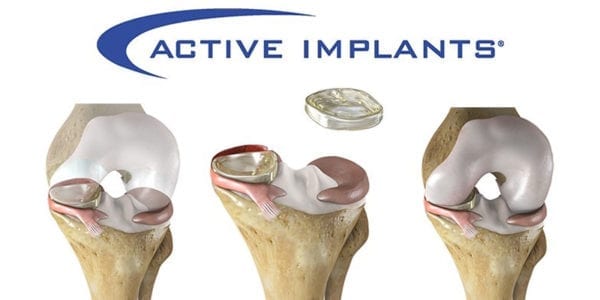
The polymer device, inserted in the knee joint through a small incision, is designed to mimic the function of the natural meniscus and redistribute loads transmitted across the joint. It does not require fixation to bone or soft tissues.
In the U.S., NUsurface was granted a Breakthrough Device Designation from FDA. The program expedites the development and review process for medical devices that are novel or offer new technology for patients with life-threatening or irreversibly debilitating conditions.
“Filling the gap in treatment options between minimally invasive meniscus repair and total knee replacement is a large unmet need in the orthopedic market,” said Ted Davis, president and CEO of Active Implants. “The NUsurface Implant was invented and developed in our R&D center in Israel, so for us, it is very exciting to finally bring the device to people in Israel.”
NUsurface is not yet FDA-cleared.
Active Implants announced that two patients in Israel have undergone knee surgery with the company’s NUsurface® Meniscus Implant, the first artificial meniscus to be marketed in the Middle East. Previously, the device was only available in Israel for clinical trials.
Studies have shown that many meniscectomy patients continue to experience...

Active Implants NUsurface – Active Implants
Active Implants announced that two patients in Israel have undergone knee surgery with the company’s NUsurface® Meniscus Implant, the first artificial meniscus to be marketed in the Middle East. Previously, the device was only available in Israel for clinical trials.
Studies have shown that many meniscectomy patients continue to experience pain that affects the quality of life and can lead to knee replacement. Transplant tissue is scarce, and waiting lists exist in many countries. NUsurface offers an alternative.
The polymer device, inserted in the knee joint through a small incision, is designed to mimic the function of the natural meniscus and redistribute loads transmitted across the joint. It does not require fixation to bone or soft tissues.
In the U.S., NUsurface was granted a Breakthrough Device Designation from FDA. The program expedites the development and review process for medical devices that are novel or offer new technology for patients with life-threatening or irreversibly debilitating conditions.
“Filling the gap in treatment options between minimally invasive meniscus repair and total knee replacement is a large unmet need in the orthopedic market,” said Ted Davis, president and CEO of Active Implants. “The NUsurface Implant was invented and developed in our R&D center in Israel, so for us, it is very exciting to finally bring the device to people in Israel.”
NUsurface is not yet FDA-cleared.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







