
 Copy to clipboard
Copy to clipboard 
Active Implants was granted a Breakthrough Device Designation from FDA for the NUsurface® Meniscus Implant. NUsurface is the first artificial meniscus to be marketed in Europe and, if cleared by FDA, would be the first artificial meniscus in the U.S.
FDA’s Breakthrough Devices Program was created to expedite the development and review process for medical devices that are novel or offer new technology for patients with life-threatening or irreversibly debilitating conditions. It is designed to ensure that patients and healthcare providers have timely access to vital devices.
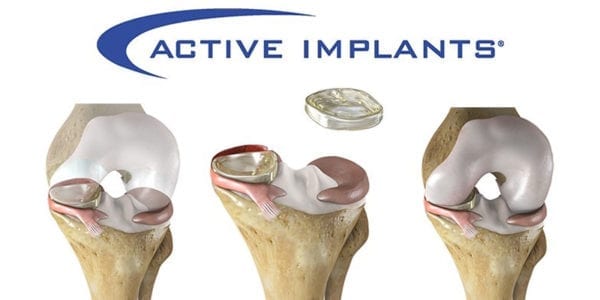
NUsurface is an investigational treatment in the U.S. for patients with persistent knee pain following medial meniscus surgery. Made from polymer and as a result of its materials, composite structure and design, it does not require fixation to bone or soft tissue. The implant mimics the function of the natural meniscus and redistributes loads across the knee joint.
“We believe we will have the data required for our FDA submission next year, after completing enrollment in our clinical trials in 2018,” said Ted Davis, President and CEO. “We look forward to working closely with the FDA to expedite the review process for the NUsurface® Implant to provide a new treatment option to the hundreds of thousands of patients who continue to experience persistent knee pain following meniscus surgery, but are too young for total knee replacement.”
Source: Active Implants LLC
Active Implants was granted a Breakthrough Device Designation from FDA for the NUsurface® Meniscus Implant. NUsurface is the first artificial meniscus to be marketed in Europe and, if cleared by FDA, would be the first artificial meniscus in the U.S.
FDA's Breakthrough Devices Program was created to expedite the development and review process...
Active Implants was granted a Breakthrough Device Designation from FDA for the NUsurface® Meniscus Implant. NUsurface is the first artificial meniscus to be marketed in Europe and, if cleared by FDA, would be the first artificial meniscus in the U.S.
FDA’s Breakthrough Devices Program was created to expedite the development and review process for medical devices that are novel or offer new technology for patients with life-threatening or irreversibly debilitating conditions. It is designed to ensure that patients and healthcare providers have timely access to vital devices.
NUsurface is an investigational treatment in the U.S. for patients with persistent knee pain following medial meniscus surgery. Made from polymer and as a result of its materials, composite structure and design, it does not require fixation to bone or soft tissue. The implant mimics the function of the natural meniscus and redistributes loads across the knee joint.
“We believe we will have the data required for our FDA submission next year, after completing enrollment in our clinical trials in 2018,” said Ted Davis, President and CEO. “We look forward to working closely with the FDA to expedite the review process for the NUsurface® Implant to provide a new treatment option to the hundreds of thousands of patients who continue to experience persistent knee pain following meniscus surgery, but are too young for total knee replacement.”
Source: Active Implants LLC

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







