
 Copy to clipboard
Copy to clipboard 
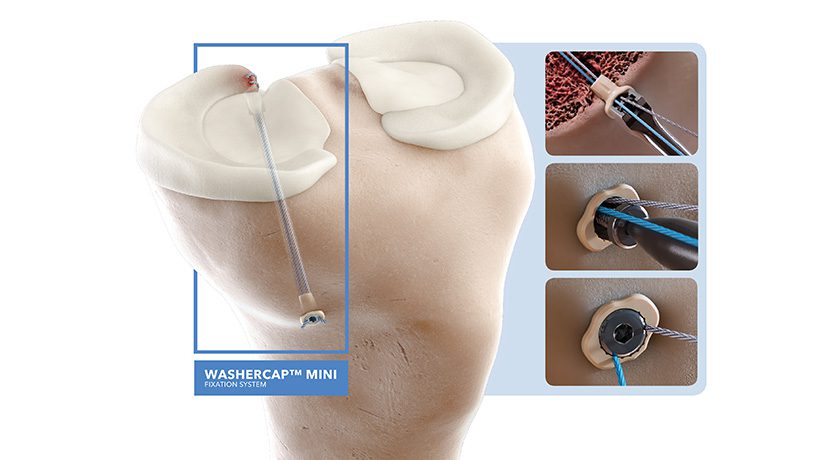
ABANZA was granted FDA 510(k) clearance for its WasherCap Mini fixation system. This device is designed for multiple applications, including meniscal root repair and ACL reconstruction.
The WasherCap Mini is the first suture and tape fixation device that offers surgeons a knotless, bidirectionally tension-adjustable solution, regardless of bone quality.
Biomechanical testing has shown the WasherCap Mini to deliver superior fixation strength and minimal displacement during cyclic loading when compared to conventional devices like cortical buttons and suture anchors.
“FDA clearance of the WasherCap Mini is a tremendous milestone for ABANZA,” said Juan Abascal, Chief Executive Officer at ABANZA. “Our device provides surgeons with a highly reliable solution for suture and tape fixation—particularly in surgeries where precise tension control is critical. For meniscal root repair, its knotless technology corrects extrusion and restores the meniscus to its anatomical position, which we believe will lead to better short and long-term outcomes, while potentially slowing the progression of arthritis.”
WasherCap Mini is the second device in a new platform of products built on disruptive technology and a shared commitment to advancing soft-tissue repair. Upcoming innovations, including the WasherCap In Line and the LoopCap, will extend ABANZA’s portfolio, offering surgeons robust solutions for procedures like biceps tenodesis, medial collateral ligament repairs, and challenging foot and ankle pathologies.
Source: ABANZA
ABANZA was granted FDA 510(k) clearance for its WasherCap Mini fixation system. This device is designed for multiple applications, including meniscal root repair and ACL reconstruction.
The WasherCap Mini is the first suture and tape fixation device that offers surgeons a knotless, bidirectionally tension-adjustable solution, regardless of...
ABANZA was granted FDA 510(k) clearance for its WasherCap Mini fixation system. This device is designed for multiple applications, including meniscal root repair and ACL reconstruction.
The WasherCap Mini is the first suture and tape fixation device that offers surgeons a knotless, bidirectionally tension-adjustable solution, regardless of bone quality.
Biomechanical testing has shown the WasherCap Mini to deliver superior fixation strength and minimal displacement during cyclic loading when compared to conventional devices like cortical buttons and suture anchors.
“FDA clearance of the WasherCap Mini is a tremendous milestone for ABANZA,” said Juan Abascal, Chief Executive Officer at ABANZA. “Our device provides surgeons with a highly reliable solution for suture and tape fixation—particularly in surgeries where precise tension control is critical. For meniscal root repair, its knotless technology corrects extrusion and restores the meniscus to its anatomical position, which we believe will lead to better short and long-term outcomes, while potentially slowing the progression of arthritis.”
WasherCap Mini is the second device in a new platform of products built on disruptive technology and a shared commitment to advancing soft-tissue repair. Upcoming innovations, including the WasherCap In Line and the LoopCap, will extend ABANZA’s portfolio, offering surgeons robust solutions for procedures like biceps tenodesis, medial collateral ligament repairs, and challenging foot and ankle pathologies.
Source: ABANZA

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







