
 Copy to clipboard
Copy to clipboard 
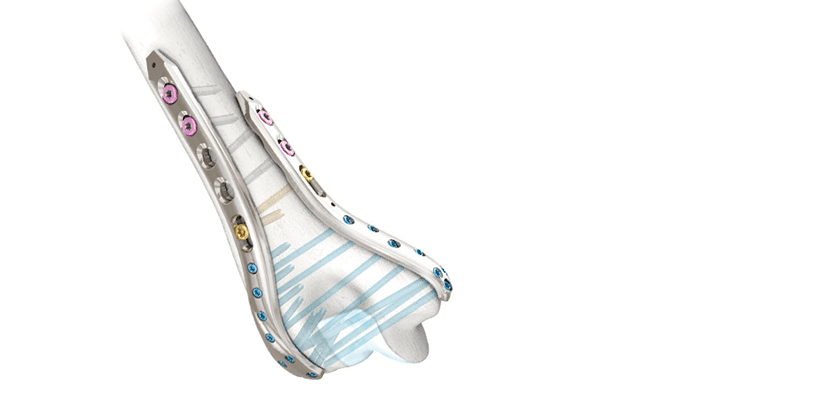
aap Implantate received FDA 510(k) clearance to market LOQTEQ VA (Variable Angle) Elbow 2.7/3.5 plates, and plans a 4Q23 launch in the U.S. and other markets.
The system supports flexible treatment of fractures of the distal humerus and olecranon important to the elbow function and stability. These are polyaxial implants that facilitate inserting angle-stable screws at different angles, to improve flexibility within the application.
The LOQTEQ VA Elbow 2.7/3.5 system comprises a variety of plate solutions for the distal humerus, enabling 90° and 180° treatment options, as well as solution for the olecranon.
With the LOQTEQ VA Elbow 2.7/3.5, aap further expands its portfolio in the polyaxial range.
Source: aap Implantate
aap Implantate received FDA 510(k) clearance to market LOQTEQ VA (Variable Angle) Elbow 2.7/3.5 plates, and plans a 4Q23 launch in the U.S. and other markets.
The system supports flexible treatment of fractures of the distal humerus and olecranon important to the elbow function and stability. These are polyaxial implants that facilitate...
aap Implantate received FDA 510(k) clearance to market LOQTEQ VA (Variable Angle) Elbow 2.7/3.5 plates, and plans a 4Q23 launch in the U.S. and other markets.
The system supports flexible treatment of fractures of the distal humerus and olecranon important to the elbow function and stability. These are polyaxial implants that facilitate inserting angle-stable screws at different angles, to improve flexibility within the application.
The LOQTEQ VA Elbow 2.7/3.5 system comprises a variety of plate solutions for the distal humerus, enabling 90° and 180° treatment options, as well as solution for the olecranon.
With the LOQTEQ VA Elbow 2.7/3.5, aap further expands its portfolio in the polyaxial range.
Source: aap Implantate

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







