Copy to clipboard
Copy to clipboard 
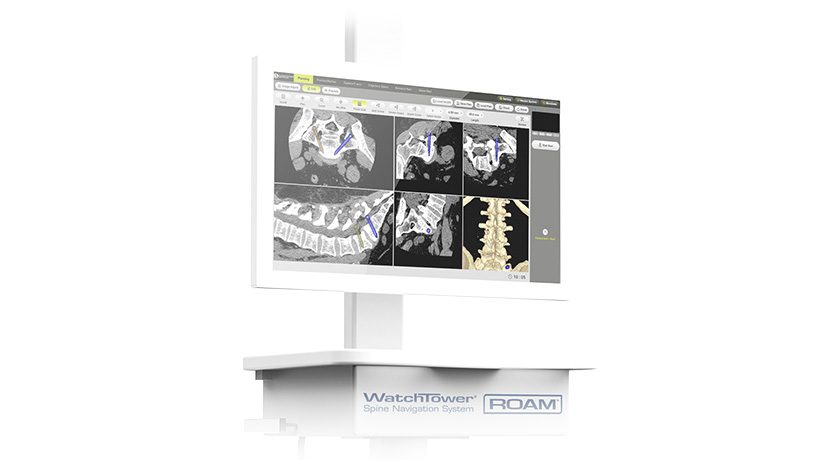
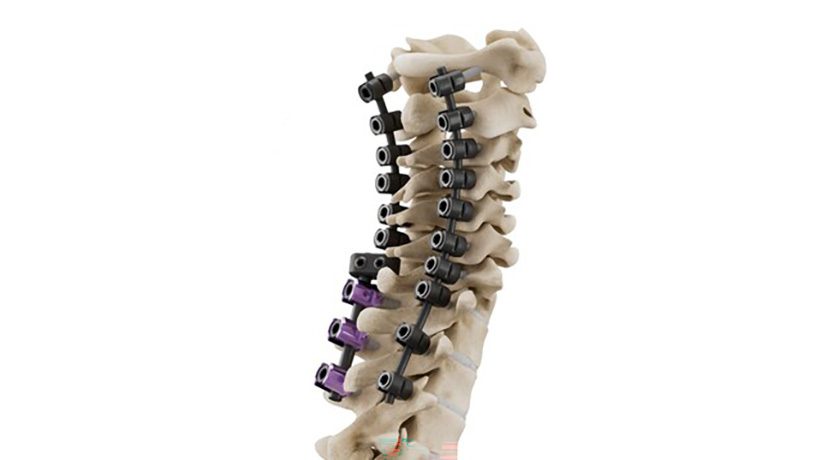
HAPPE Spine’s INTEGRATE-C Interbody Fusion System has been implanted in more than 1,000 patients, demonstrating clinical adoption of the HAPPE platform across more than 30 surgeon users and 40 facilities nationwide.
The company closed an oversubscribed bridge funding round earlier this year to support continued clinical validation of the HAPPE platform in the INTEGRATE-C Interbody Fusion System, and expansion of manufacturing capabilities to support commercial growth and future product offerings.
The device gained FDA 510(k) marketing clearance in 2023.
Source: HAPPE Spine:
HAPPE Spine's INTEGRATE-C Interbody Fusion System has been implanted in more than 1,000 patients, demonstrating clinical adoption of the HAPPE platform across more than 30 surgeon users and 40 facilities nationwide.
The company closed an oversubscribed bridge funding round earlier this year to support continued clinical validation of the HAPPE...
HAPPE Spine’s INTEGRATE-C Interbody Fusion System has been implanted in more than 1,000 patients, demonstrating clinical adoption of the HAPPE platform across more than 30 surgeon users and 40 facilities nationwide.
The company closed an oversubscribed bridge funding round earlier this year to support continued clinical validation of the HAPPE platform in the INTEGRATE-C Interbody Fusion System, and expansion of manufacturing capabilities to support commercial growth and future product offerings.
The device gained FDA 510(k) marketing clearance in 2023.
Source: HAPPE Spine:

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.