Copy to clipboard
Copy to clipboard 
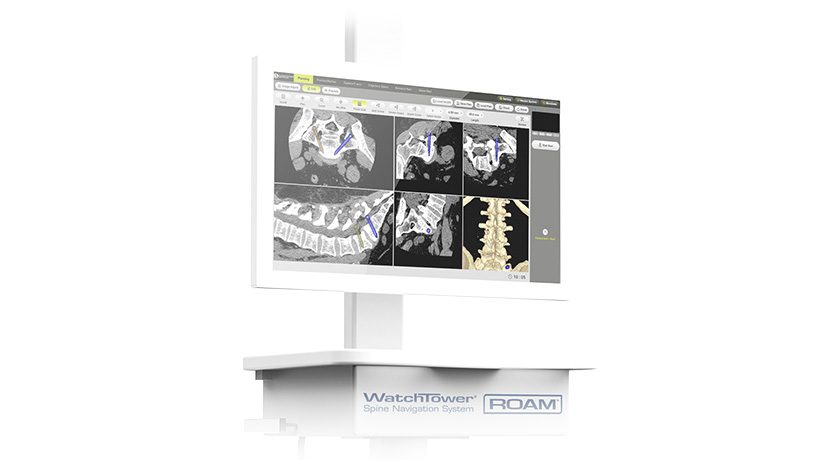
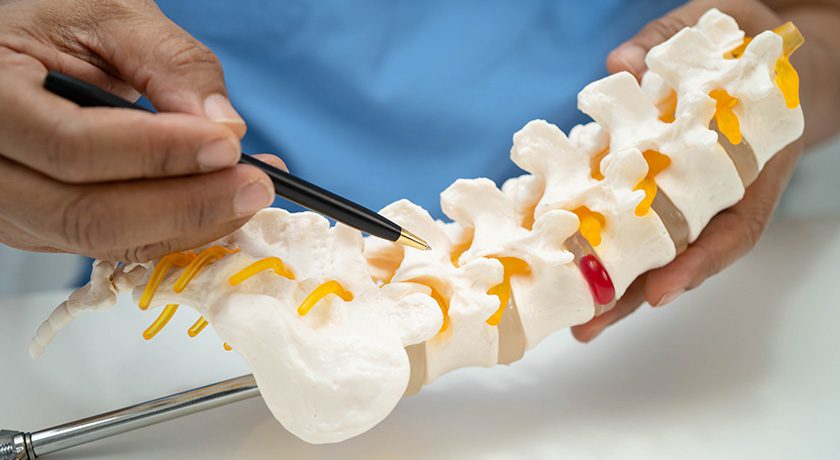
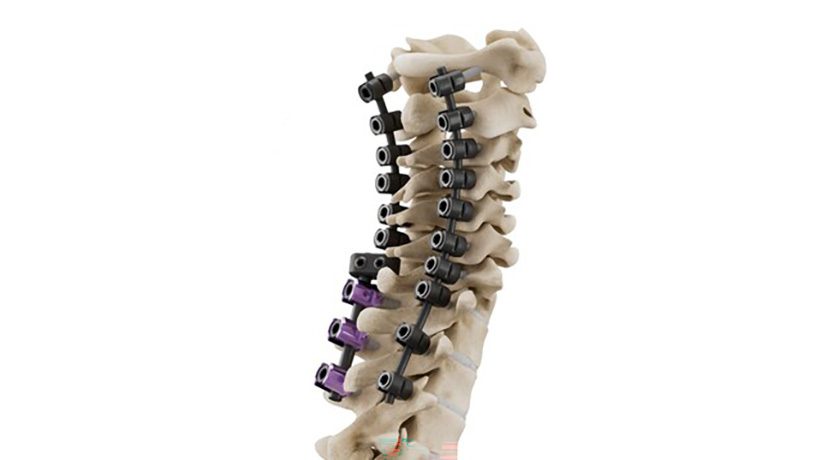
Centinel Spine marked the milestone of more than 300,000 prodisc devices implanted worldwide.
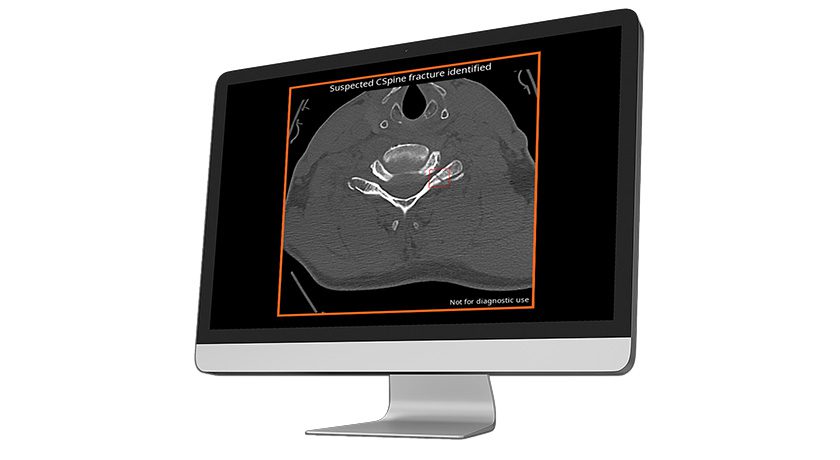
Most recently, the Company received FDA approval for two-level use of the prodiscC Vivo and prodisc C SK cervical TDR devices. Centinel Spine remains exclusively dedicated to total disc replacement, with extensive and continuous innovation planned well into the future.
“Reaching 300,000 prodisc implantations is an important milestone reflecting the trust surgeons place in total disc replacement and the meaningful impact this technology has had on patients,” concluded Steve Murray, CEO of Centinel Spine. “We remain focused on supporting surgeons, providing clinically proven solutions, and advancing total disc replacement as an important treatment option for patients with cervical and lumbar spinal disease.”
Source: Centinel Spine, LLC
Centinel Spine marked the milestone of more than 300,000 prodisc devices implanted worldwide.
Most recently, the Company received FDA approval for two-level use of the prodiscC Vivo and prodisc C SK cervical TDR devices. Centinel Spine remains exclusively dedicated to total disc replacement, with extensive and continuous innovation planned...
Centinel Spine marked the milestone of more than 300,000 prodisc devices implanted worldwide.
Most recently, the Company received FDA approval for two-level use of the prodiscC Vivo and prodisc C SK cervical TDR devices. Centinel Spine remains exclusively dedicated to total disc replacement, with extensive and continuous innovation planned well into the future.
“Reaching 300,000 prodisc implantations is an important milestone reflecting the trust surgeons place in total disc replacement and the meaningful impact this technology has had on patients,” concluded Steve Murray, CEO of Centinel Spine. “We remain focused on supporting surgeons, providing clinically proven solutions, and advancing total disc replacement as an important treatment option for patients with cervical and lumbar spinal disease.”
Source: Centinel Spine, LLC

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.