Copy to clipboard
Copy to clipboard 
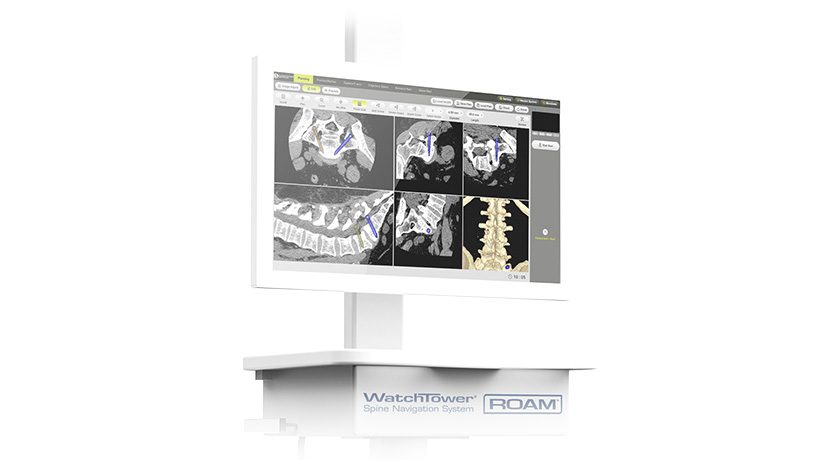
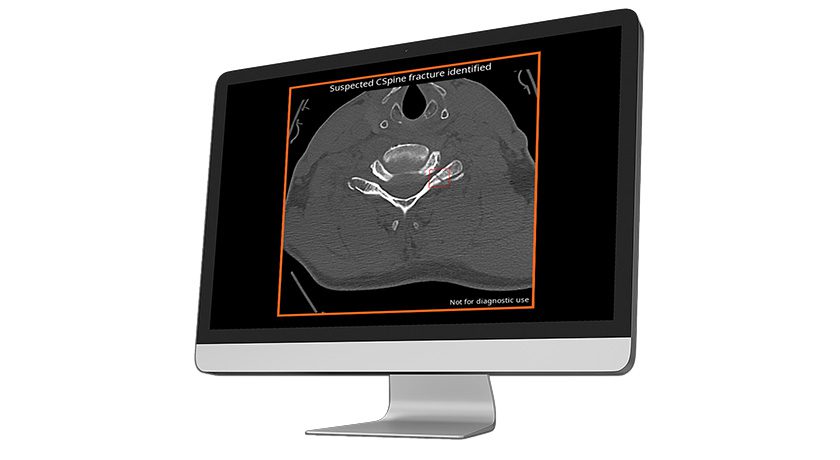
Hubly Surgical received FDA 510(k) clearance expanding Hubly Auto-Stop Drill indications to include spinal decompression procedures. In neurosurgical applications, the device’s SMART auto-stop halts rotation and prevents forward plunge at the instant of skull penetration, preventing over-drilling into patients’ brains. The new clearance expands use to laminectomy and laminotomy for spinal decompression to drill through the vertebral lamina and protect patients from damage to the spinal cord.
The single-use, cordless Hubly Auto-Stop Drill features SMART auto-stop that detects breakthrough and immediately stops rotation upon bone penetration. This proprietary mechanism is combined with mechanical plunge-prevention to prevent forward movement of the drill upon this auto-stop and protect the patient from harm, as well as real-time visual force indication that aids user control. These design elements are intended to enhance safety, speed, and efficiency and streamline setup and workflow compared with today’s standard of care.
“Today’s clearance marks a pivotal step for Hubly’s platform,” said Casey Qadir, Founder & CEO of Hubly Surgical. “Our goal is to save and improve lives by giving surgeons intuitive tools that protect critical tissue every single time. With cranial and spinal indications now in hand, we can support neuro-spine teams across the board. One consistent, safe device experience across settings and use cases.”
Source: Hubly Surgical
Hubly Surgical received FDA 510(k) clearance expanding Hubly Auto-Stop Drill indications to include spinal decompression procedures. In neurosurgical applications, the device's SMART auto-stop halts rotation and prevents forward plunge at the instant of skull penetration, preventing over-drilling into patients' brains. The new clearance expands...
Hubly Surgical received FDA 510(k) clearance expanding Hubly Auto-Stop Drill indications to include spinal decompression procedures. In neurosurgical applications, the device’s SMART auto-stop halts rotation and prevents forward plunge at the instant of skull penetration, preventing over-drilling into patients’ brains. The new clearance expands use to laminectomy and laminotomy for spinal decompression to drill through the vertebral lamina and protect patients from damage to the spinal cord.
The single-use, cordless Hubly Auto-Stop Drill features SMART auto-stop that detects breakthrough and immediately stops rotation upon bone penetration. This proprietary mechanism is combined with mechanical plunge-prevention to prevent forward movement of the drill upon this auto-stop and protect the patient from harm, as well as real-time visual force indication that aids user control. These design elements are intended to enhance safety, speed, and efficiency and streamline setup and workflow compared with today’s standard of care.
“Today’s clearance marks a pivotal step for Hubly’s platform,” said Casey Qadir, Founder & CEO of Hubly Surgical. “Our goal is to save and improve lives by giving surgeons intuitive tools that protect critical tissue every single time. With cranial and spinal indications now in hand, we can support neuro-spine teams across the board. One consistent, safe device experience across settings and use cases.”
Source: Hubly Surgical

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.