
 Copy to clipboard
Copy to clipboard 
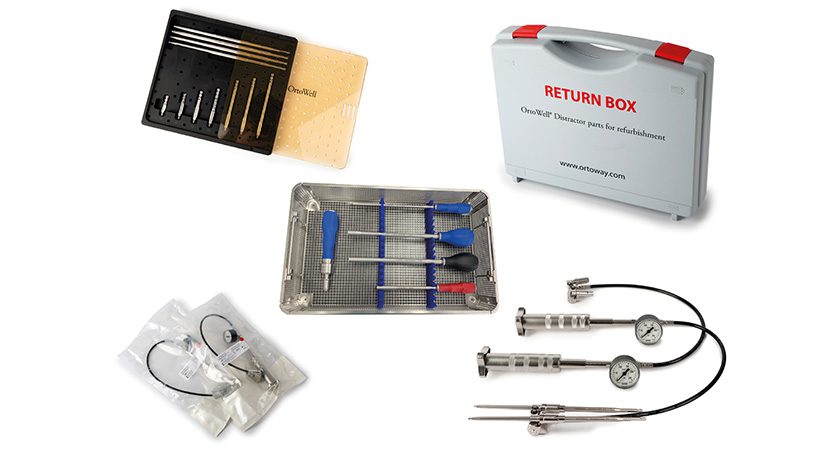
OrtoWay-US Inc announced the publication of the first prospective, multi-center clinical study evaluating its OrtoWell Distractor System – the world’s first hydraulically powered vertebral distractor, according to the company.
Unlike conventional instruments such as mallets or torque-based retractors, the OrtoWell system uses a hydraulic mechanism that allows surgeons to open the vertebrae smoothly and safely, with finely controlled incremental pressure. This precise, stable control helps reduce the risk of over-distraction, complications or neural damage, leading to safer, more predictable surgical outcomes.
The study, published in Medical Devices: Evidence and Research confirms the safety, usability, and intraoperative performance of the device across 30 patients treated in Germany. Results showed no device-related complications and consistent positive surgeon feedback on precision, stability and control.
“The data confirms what surgeons have long reported – that hydraulic distraction offers a gentler, more controllable approach to vertebral separation than mechanical systems,” said Stan Mikulowski, CEO of OrtoWay-US Inc. “The OrtoWell Distractor gives surgeons a new level of tactile precision, helping reduce procedural risk while improving predictability in spinal reconstruction. This is a key step to establishing a new future standard in spinal surgery.”
Source: OrtoWay-US Inc
OrtoWay-US Inc announced the publication of the first prospective, multi-center clinical study evaluating its OrtoWell Distractor System – the world's first hydraulically powered vertebral distractor, according to the company.
Unlike conventional instruments such as mallets or torque-based retractors, the OrtoWell system uses a hydraulic...
OrtoWay-US Inc announced the publication of the first prospective, multi-center clinical study evaluating its OrtoWell Distractor System – the world’s first hydraulically powered vertebral distractor, according to the company.
Unlike conventional instruments such as mallets or torque-based retractors, the OrtoWell system uses a hydraulic mechanism that allows surgeons to open the vertebrae smoothly and safely, with finely controlled incremental pressure. This precise, stable control helps reduce the risk of over-distraction, complications or neural damage, leading to safer, more predictable surgical outcomes.
The study, published in Medical Devices: Evidence and Research confirms the safety, usability, and intraoperative performance of the device across 30 patients treated in Germany. Results showed no device-related complications and consistent positive surgeon feedback on precision, stability and control.
“The data confirms what surgeons have long reported – that hydraulic distraction offers a gentler, more controllable approach to vertebral separation than mechanical systems,” said Stan Mikulowski, CEO of OrtoWay-US Inc. “The OrtoWell Distractor gives surgeons a new level of tactile precision, helping reduce procedural risk while improving predictability in spinal reconstruction. This is a key step to establishing a new future standard in spinal surgery.”
Source: OrtoWay-US Inc

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
PM
Patrick McGuire is an ORTHOWORLD Contributor.