
 Copy to clipboard
Copy to clipboard 
ReVivo Medical successfully completed the 50th surgical procedure using the company’s anterior cervical plates and interbody cages.
“We were required to perform 50 surgical procedures as part of our FDA-sanctioned IDE clinical trial, a requirement for attaining regulatory clearance prior to offering a commercial product,” explained Gary Mittleman, President and CEO. “Although final clearance requires us to track patients for two years post-surgery, we are very pleased with our device’s performance thus far.”
“Anterior cervical plates and interbody cages are used in over 400,000 surgical procedures each year representing a multi-billion-dollar market,” said Dr. Darryl DiRisio, Chief Medical Officer. “The primary measure of success in these operations is the rapid achievement of bone fusion which thereby stabilizes the spine and eliminates pain.”
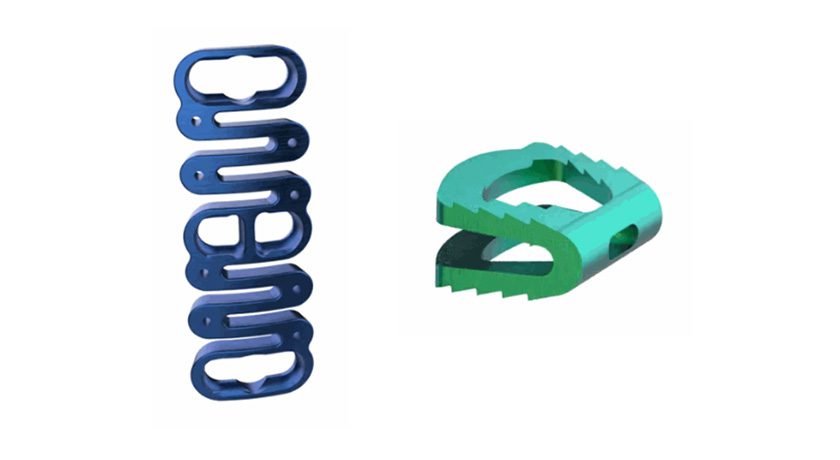
Study participants received ReVivo Medical’s next generation CEM-Plate and CEM-Cage used in anterior cervical discectomy and fusion procedures.
“Through the clinical trial, we hope to demonstrate that our cervical plate and cage implants improve patient outcomes and provide advantages for the surgeon,” explains Eric Ledet, Ph.D., Chief Operating Officer. “We plan to report our complete findings to the FDA in late 2027.”
Source: ReVivo Medical, Inc.
ReVivo Medical successfully completed the 50th surgical procedure using the company’s anterior cervical plates and interbody cages.
“We were required to perform 50 surgical procedures as part of our FDA-sanctioned IDE clinical trial, a requirement for attaining regulatory clearance prior to offering a commercial product,” explained Gary...
ReVivo Medical successfully completed the 50th surgical procedure using the company’s anterior cervical plates and interbody cages.
“We were required to perform 50 surgical procedures as part of our FDA-sanctioned IDE clinical trial, a requirement for attaining regulatory clearance prior to offering a commercial product,” explained Gary Mittleman, President and CEO. “Although final clearance requires us to track patients for two years post-surgery, we are very pleased with our device’s performance thus far.”
“Anterior cervical plates and interbody cages are used in over 400,000 surgical procedures each year representing a multi-billion-dollar market,” said Dr. Darryl DiRisio, Chief Medical Officer. “The primary measure of success in these operations is the rapid achievement of bone fusion which thereby stabilizes the spine and eliminates pain.”
Study participants received ReVivo Medical’s next generation CEM-Plate and CEM-Cage used in anterior cervical discectomy and fusion procedures.
“Through the clinical trial, we hope to demonstrate that our cervical plate and cage implants improve patient outcomes and provide advantages for the surgeon,” explains Eric Ledet, Ph.D., Chief Operating Officer. “We plan to report our complete findings to the FDA in late 2027.”
Source: ReVivo Medical, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.