
 Copy to clipboard
Copy to clipboard 
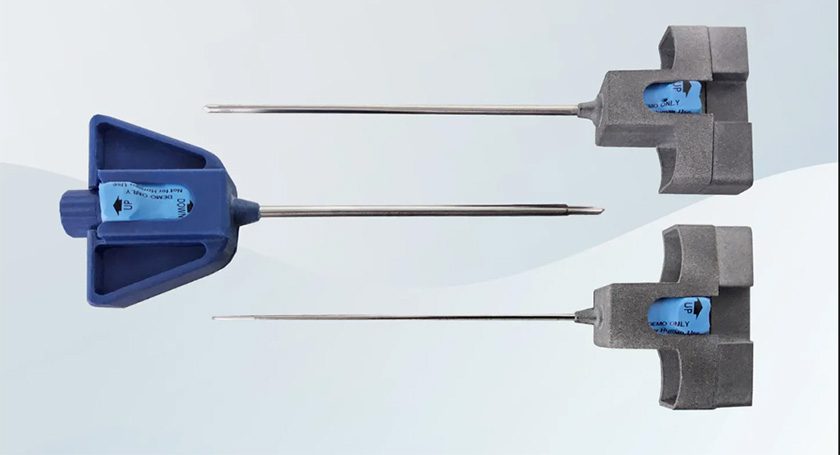
Ruthless Spine received FDA 510(k) clearance for the NavJam Jamshidi device, and U.S. Patent Office records indicate that the status of the NavJam Jamshidi patent application is allowed. The NavJam Jamshidi device is also covered by an issued Taiwan patent and is designed to work in tandem with Ruthless Spine’s De Novo cleared RJB intraoperative angle measurement instrument.
The NavJam offers an alternative to bulky, capital-intensive traditional navigation. By simplifying workflow, reducing surgical time, and eliminating costly infrastructure, the NavJam and RJB together provide a scalable solution to one of the most pressing challenges in modern spine surgery: significantly minimizing radiation and maintaining precision while lowering time and cost.
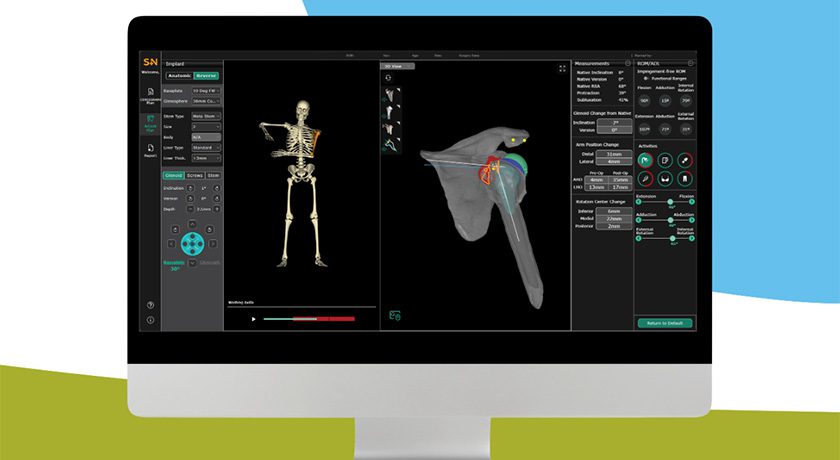
Ruthless Spine’s platform originated from the idea that an alternative to traditional navigation could be possible. The RJB, cleared in 2023 through the FDA De Novo pathway, provided the first proof point: a single-use, Bluetooth-connected module that pairs with a computer tablet to display instrument orientation in real time. By eliminating capital equipment, service contracts, and long setup times, the RJB reduced average surgical time by 77 minutes per case while matching the accuracy standards of traditional navigation.
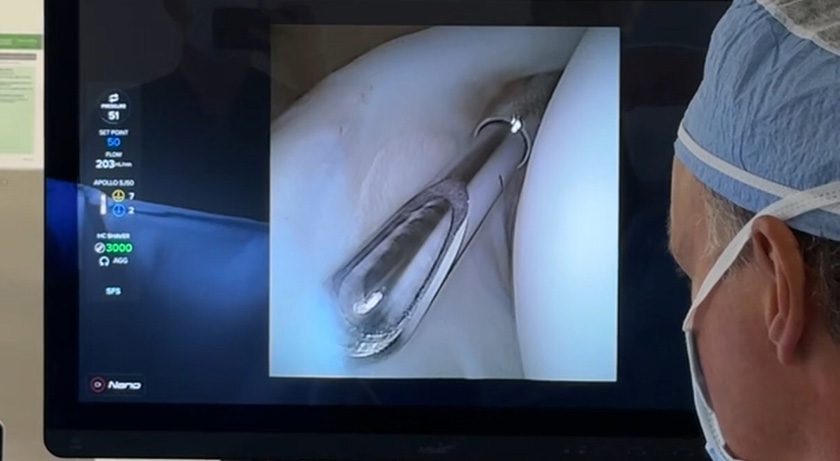
NavJam expands this concept into the Jamshidi needle step of lumbosacral pedicle screw placement. Like the RJB, it is disposable, pairs wirelessly with a tablet, and provides surgeons with real-time trajectory feedback. The device functions as a guide, supporting surgical judgment rather than replacing it.
Because the platform requires no capital investment or specialized staff, it is particularly suited for healthcare environments where multimillion-dollar navigation is not a viable option. In addition to the United States, NavJam and the RJB are already being used in Turkey, Ukraine, and Africa. “Spinal surgery is not limited to well-funded centers in the United States and Europe,” said Karlton Spindle of Ruthless Spine. “By keeping the technology simple, portable, and low-cost, we can make accurate screw placement available in regions where large systems could never be deployed.”
Source: Ruthless Spine
Ruthless Spine received FDA 510(k) clearance for the NavJam Jamshidi device, and U.S. Patent Office records indicate that the status of the NavJam Jamshidi patent application is allowed. The NavJam Jamshidi device is also covered by an issued Taiwan patent and is designed to work in tandem with Ruthless Spine’s De Novo cleared RJB intraoperative...
Ruthless Spine received FDA 510(k) clearance for the NavJam Jamshidi device, and U.S. Patent Office records indicate that the status of the NavJam Jamshidi patent application is allowed. The NavJam Jamshidi device is also covered by an issued Taiwan patent and is designed to work in tandem with Ruthless Spine’s De Novo cleared RJB intraoperative angle measurement instrument.
The NavJam offers an alternative to bulky, capital-intensive traditional navigation. By simplifying workflow, reducing surgical time, and eliminating costly infrastructure, the NavJam and RJB together provide a scalable solution to one of the most pressing challenges in modern spine surgery: significantly minimizing radiation and maintaining precision while lowering time and cost.
Ruthless Spine’s platform originated from the idea that an alternative to traditional navigation could be possible. The RJB, cleared in 2023 through the FDA De Novo pathway, provided the first proof point: a single-use, Bluetooth-connected module that pairs with a computer tablet to display instrument orientation in real time. By eliminating capital equipment, service contracts, and long setup times, the RJB reduced average surgical time by 77 minutes per case while matching the accuracy standards of traditional navigation.
NavJam expands this concept into the Jamshidi needle step of lumbosacral pedicle screw placement. Like the RJB, it is disposable, pairs wirelessly with a tablet, and provides surgeons with real-time trajectory feedback. The device functions as a guide, supporting surgical judgment rather than replacing it.
Because the platform requires no capital investment or specialized staff, it is particularly suited for healthcare environments where multimillion-dollar navigation is not a viable option. In addition to the United States, NavJam and the RJB are already being used in Turkey, Ukraine, and Africa. “Spinal surgery is not limited to well-funded centers in the United States and Europe,” said Karlton Spindle of Ruthless Spine. “By keeping the technology simple, portable, and low-cost, we can make accurate screw placement available in regions where large systems could never be deployed.”
Source: Ruthless Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.