Copy to clipboard
Copy to clipboard 
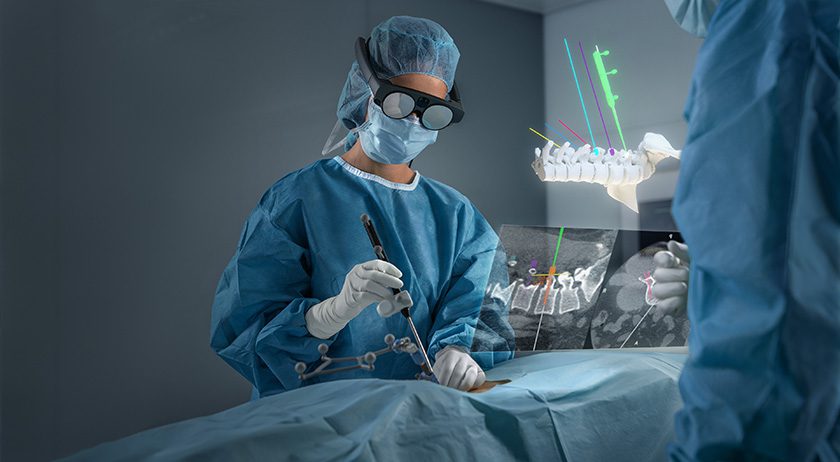
Orthopedic Driven Imaging (ODI) announced FDA 510(k) clearance to market its surgical c-arm system, S-ODI See-arm, developed in partnership with Imaging Engineering.
By combining novel low-dose fluoroscopic hardware with an AI-enabled imaging solution, ODI addresses limitations of traditional 2D x-ray and high-cost CT systems. The company’s ecosystem includes tools for 3D surgical planning, intraoperative guidance, and postoperative evaluation, all tailored to the unique anatomy and motion of each patient.
ODI’s initial product portfolio of fluoroscopic systems includes a surgical ODI unit (S-ODI), a clinical unit for extremities (C-ODI x), a clinical unit for single joint imaging and arthrograms (C-ODI Lite), and a suite of associated AI-powered surgical apps. The initial value of ODI’s technology will be evident in procedures such as total hip replacement, where enhanced imaging of the pelvis improves rotational accuracy and implant positioning.
ODI’s innovations are built on decades of scientific research, including dynamic kinematic analysis pioneered by Scott Banks, PhD, co-founder of ODI and Knee Society member. “With ODI, we are re-engineering advanced imaging and analysis, giving surgeons practical tools they can use with their patients across the entire continuum of care,” said Dr. Banks. The company’s platform will close the information loop for surgeons, providing quantitative, real-time, dynamic 3D insights (4D) before, during, and after orthopedic procedures.
“Obtaining FDA 510(k) clearance for the S-ODI platform marks a defining moment for ODI and, we believe, for the future of orthopedic imaging,” said Don Running, ODI’s Chief Executive Officer. “This milestone marks the start of ODI’s development of a fully integrated imaging platform for orthopedic surgery.”
Source: Orthopedic Driven Imaging (ODI)
Orthopedic Driven Imaging (ODI) announced FDA 510(k) clearance to market its surgical c-arm system, S-ODI See-arm, developed in partnership with Imaging Engineering.
By combining novel low-dose fluoroscopic hardware with an AI-enabled imaging solution, ODI addresses limitations of traditional 2D x-ray and high-cost CT systems. The company’s...
Orthopedic Driven Imaging (ODI) announced FDA 510(k) clearance to market its surgical c-arm system, S-ODI See-arm, developed in partnership with Imaging Engineering.
By combining novel low-dose fluoroscopic hardware with an AI-enabled imaging solution, ODI addresses limitations of traditional 2D x-ray and high-cost CT systems. The company’s ecosystem includes tools for 3D surgical planning, intraoperative guidance, and postoperative evaluation, all tailored to the unique anatomy and motion of each patient.
ODI’s initial product portfolio of fluoroscopic systems includes a surgical ODI unit (S-ODI), a clinical unit for extremities (C-ODI x), a clinical unit for single joint imaging and arthrograms (C-ODI Lite), and a suite of associated AI-powered surgical apps. The initial value of ODI’s technology will be evident in procedures such as total hip replacement, where enhanced imaging of the pelvis improves rotational accuracy and implant positioning.
ODI’s innovations are built on decades of scientific research, including dynamic kinematic analysis pioneered by Scott Banks, PhD, co-founder of ODI and Knee Society member. “With ODI, we are re-engineering advanced imaging and analysis, giving surgeons practical tools they can use with their patients across the entire continuum of care,” said Dr. Banks. The company’s platform will close the information loop for surgeons, providing quantitative, real-time, dynamic 3D insights (4D) before, during, and after orthopedic procedures.
“Obtaining FDA 510(k) clearance for the S-ODI platform marks a defining moment for ODI and, we believe, for the future of orthopedic imaging,” said Don Running, ODI’s Chief Executive Officer. “This milestone marks the start of ODI’s development of a fully integrated imaging platform for orthopedic surgery.”
Source: Orthopedic Driven Imaging (ODI)

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.