
 Copy to clipboard
Copy to clipboard 
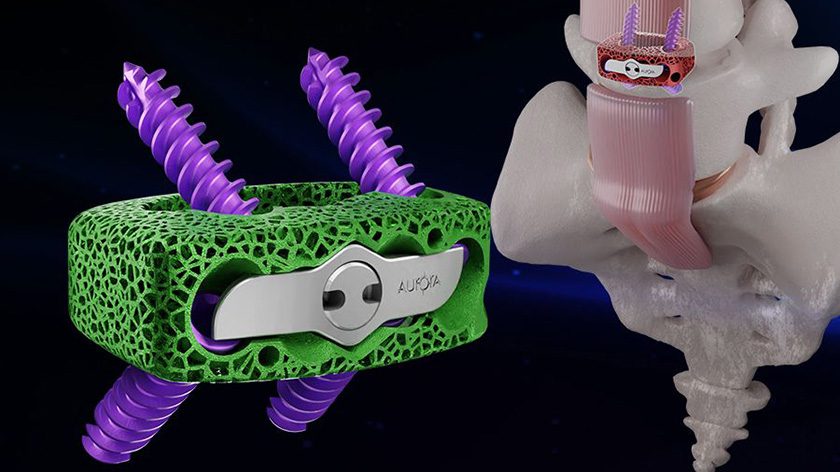
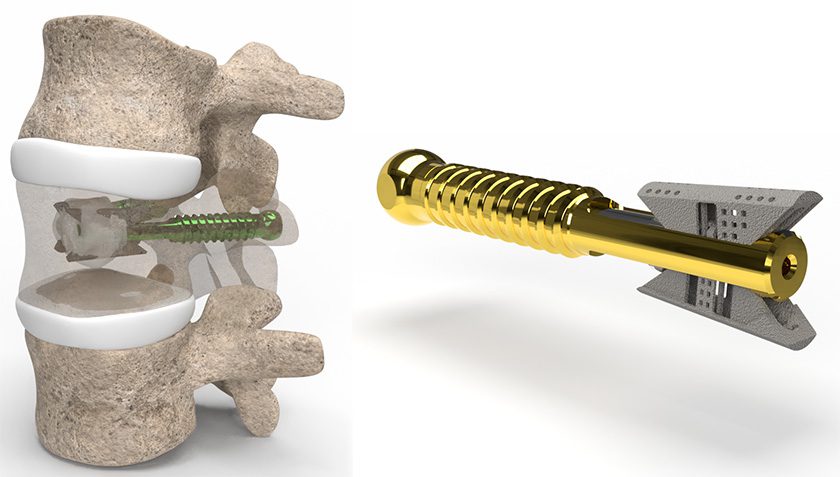
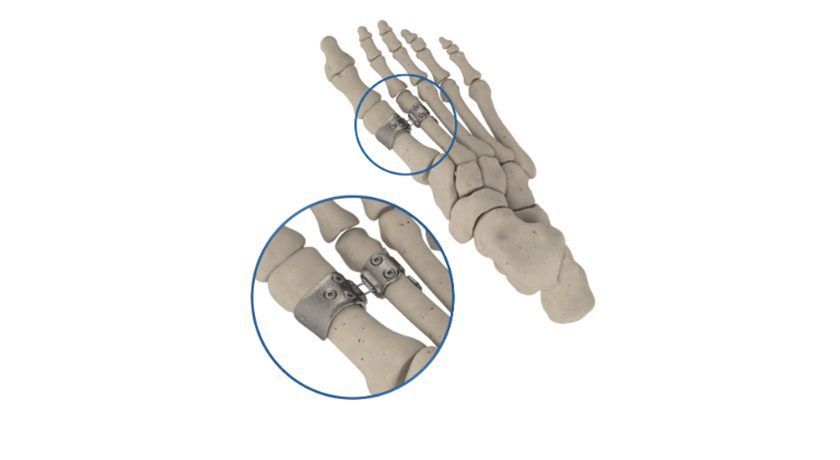
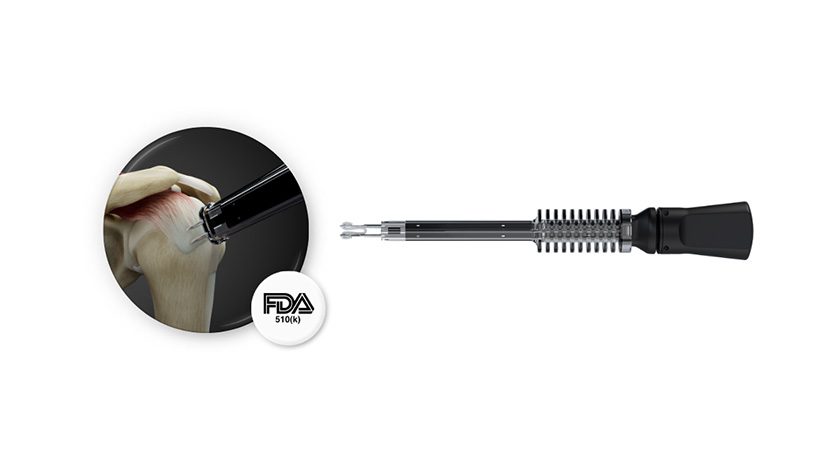
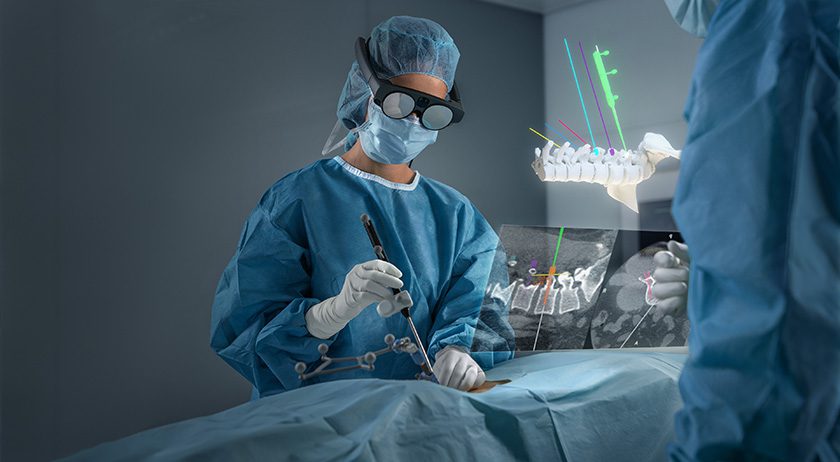
Brainlab announced FDA 510(k) marketing clearance and U.S. launch for Spine Mixed Reality Navigation. This solution delivers an ergonomic view of the navigation screen and allows surgeons to place pedicle screws with vital information directly in their field of view.
Spine Mixed Reality Navigation combines the company’s optical navigation with advanced mixed reality. This extended visualization is projected directly in the sterile field, delivering a layer of confidence one step beyond traditional technology. With real-time visualization of entry and target points, Brainlab Spine Mixed Reality Navigation enables the desired placement of pedicle screws according to the surgeon’s pre- or intraoperative plans, including in MIS cases where target identification is challenging.
“We’re ushering in the future of spine surgery, putting groundbreaking tools in the hands of US surgeons,” said Sean Clark, President, Brainlab, Inc. “European surgeons are already experiencing the power to see, critically observe and optimize clinical procedures with Spine Mixed Reality Navigation. This solution delivers hyper realistic 3D visuals and extended visualization, while the workflow, instruments and navigation provide familiar and expected precision. Together with our customers, we’re driving accuracy in the O.R.”
Source: Brainlab
Brainlab announced FDA 510(k) marketing clearance and U.S. launch for Spine Mixed Reality Navigation. This solution delivers an ergonomic view of the navigation screen and allows surgeons to place pedicle screws with vital information directly in their field of view.
Spine Mixed Reality Navigation combines the company’s optical navigation with...
Brainlab announced FDA 510(k) marketing clearance and U.S. launch for Spine Mixed Reality Navigation. This solution delivers an ergonomic view of the navigation screen and allows surgeons to place pedicle screws with vital information directly in their field of view.
Spine Mixed Reality Navigation combines the company’s optical navigation with advanced mixed reality. This extended visualization is projected directly in the sterile field, delivering a layer of confidence one step beyond traditional technology. With real-time visualization of entry and target points, Brainlab Spine Mixed Reality Navigation enables the desired placement of pedicle screws according to the surgeon’s pre- or intraoperative plans, including in MIS cases where target identification is challenging.
“We’re ushering in the future of spine surgery, putting groundbreaking tools in the hands of US surgeons,” said Sean Clark, President, Brainlab, Inc. “European surgeons are already experiencing the power to see, critically observe and optimize clinical procedures with Spine Mixed Reality Navigation. This solution delivers hyper realistic 3D visuals and extended visualization, while the workflow, instruments and navigation provide familiar and expected precision. Together with our customers, we’re driving accuracy in the O.R.”
Source: Brainlab

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.