
 Copy to clipboard
Copy to clipboard 
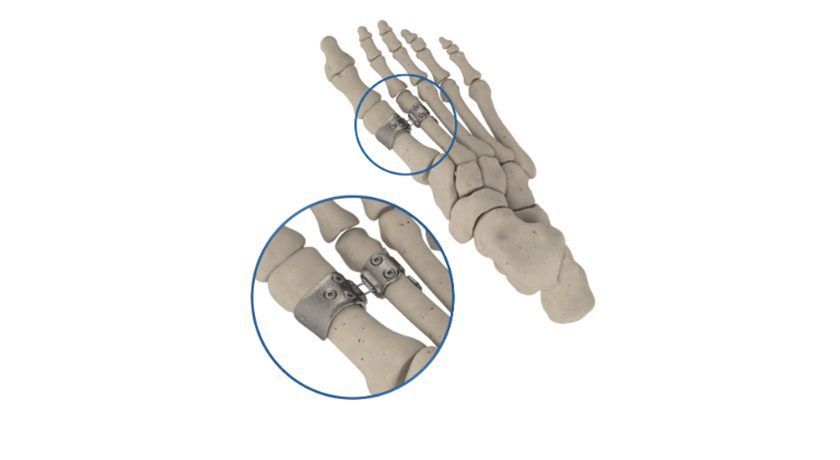
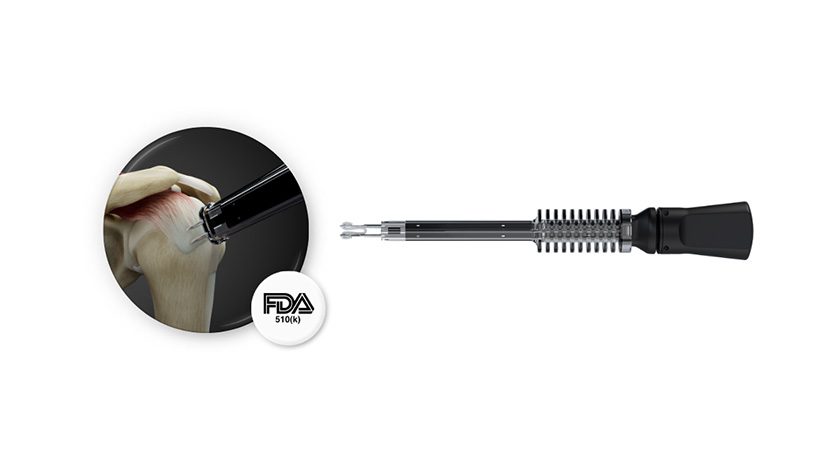
SutureTech was granted FDA 510(k) clearance to market RapidFix, an All-Suture Dual Anchor System. RapidFix is intended for fixation of soft tissue to bone in a variety of orthopedic procedures. Limited launch will occur within 4Q25.
RapidFix is a 100% suture-based internal fixation device engineered to create secure tendon-to-bone repairs while reducing procedural steps and surgical complexity. Developed through eight years of surgeon-led collaboration, the device has been validated through preclinical testing for safety and performance.
“Achieving FDA clearance for RapidFix is a defining moment for SutureTech,” said Dr. Oke Anakwenze, Founder & CEO of SutureTech and Chief of Shoulder Surgery at Duke University. “The device was designed to streamline surgical steps while maintaining strong, reliable fixation. We believe RapidFix offers value for surgeons seeking reproducibility—and for patients who benefit from more consistent repairs.”
Source: SutureTech
SutureTech was granted FDA 510(k) clearance to market RapidFix, an All-Suture Dual Anchor System. RapidFix is intended for fixation of soft tissue to bone in a variety of orthopedic procedures. Limited launch will occur within 4Q25.
RapidFix is a 100% suture-based internal fixation device engineered to create secure tendon-to-bone repairs...
SutureTech was granted FDA 510(k) clearance to market RapidFix, an All-Suture Dual Anchor System. RapidFix is intended for fixation of soft tissue to bone in a variety of orthopedic procedures. Limited launch will occur within 4Q25.
RapidFix is a 100% suture-based internal fixation device engineered to create secure tendon-to-bone repairs while reducing procedural steps and surgical complexity. Developed through eight years of surgeon-led collaboration, the device has been validated through preclinical testing for safety and performance.
“Achieving FDA clearance for RapidFix is a defining moment for SutureTech,” said Dr. Oke Anakwenze, Founder & CEO of SutureTech and Chief of Shoulder Surgery at Duke University. “The device was designed to streamline surgical steps while maintaining strong, reliable fixation. We believe RapidFix offers value for surgeons seeking reproducibility—and for patients who benefit from more consistent repairs.”
Source: SutureTech

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.