
 Copy to clipboard
Copy to clipboard 
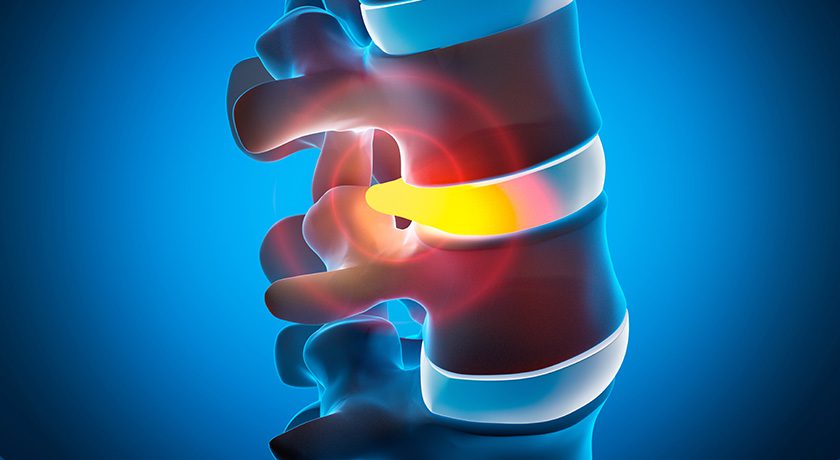
Vivex Biologics has completed subject enrollment in its ASCEND clinical trial evaluating VIA Disc NP for symptomatic degenerative disc disease (DDD).
This randomized, double-blind, sham-controlled trial enrolled 110 participants across five sites in Australia to assess the safety and efficacy of VIA Disc NP versus a sham procedure in subjects suffering lumbar discogenic pain associated with moderate to severe DDD. The primary endpoint of the trial is the proportion of participants achieving a minimal clinically important difference (MCID) in back pain visual analogue scale (VAS) score, defined as a 30% reduction from baseline at six months post-procedure. Data from the trial are expected in the first quarter of 2026.
In addition, Vivex is enrolling participants 22-85 years of age in an additional Level 1, randomized, sham-controlled trial known as RESTORE in the United States. This is an expanded clinical investigation of VIA Disc NP, conducted at up to 20 clinical sites, that will pave the way for further insights to strengthen the growing body of evidence supporting VIA Disc NP.
“We are pleased to announce the completion of enrollment in our ASCEND trial ahead of schedule, a key milestone in the continued development of VIA Disc NP,” said Barry Salzman, Co-President and Chief Operating Officer of Vivex. “This achievement reflects the dedication of our clinical teams and strong engagement from the spine care community, underscoring the need for innovative, minimally invasive treatment options for DDD.”
Source: Vivex Biologics, Inc.
Vivex Biologics has completed subject enrollment in its ASCEND clinical trial evaluating VIA Disc NP for symptomatic degenerative disc disease (DDD).
This randomized, double-blind, sham-controlled trial enrolled 110 participants across five sites in Australia to assess the safety and efficacy of VIA Disc NP versus a sham procedure in subjects...
Vivex Biologics has completed subject enrollment in its ASCEND clinical trial evaluating VIA Disc NP for symptomatic degenerative disc disease (DDD).
This randomized, double-blind, sham-controlled trial enrolled 110 participants across five sites in Australia to assess the safety and efficacy of VIA Disc NP versus a sham procedure in subjects suffering lumbar discogenic pain associated with moderate to severe DDD. The primary endpoint of the trial is the proportion of participants achieving a minimal clinically important difference (MCID) in back pain visual analogue scale (VAS) score, defined as a 30% reduction from baseline at six months post-procedure. Data from the trial are expected in the first quarter of 2026.
In addition, Vivex is enrolling participants 22-85 years of age in an additional Level 1, randomized, sham-controlled trial known as RESTORE in the United States. This is an expanded clinical investigation of VIA Disc NP, conducted at up to 20 clinical sites, that will pave the way for further insights to strengthen the growing body of evidence supporting VIA Disc NP.
“We are pleased to announce the completion of enrollment in our ASCEND trial ahead of schedule, a key milestone in the continued development of VIA Disc NP,” said Barry Salzman, Co-President and Chief Operating Officer of Vivex. “This achievement reflects the dedication of our clinical teams and strong engagement from the spine care community, underscoring the need for innovative, minimally invasive treatment options for DDD.”
Source: Vivex Biologics, Inc.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.