
 Copy to clipboard
Copy to clipboard 
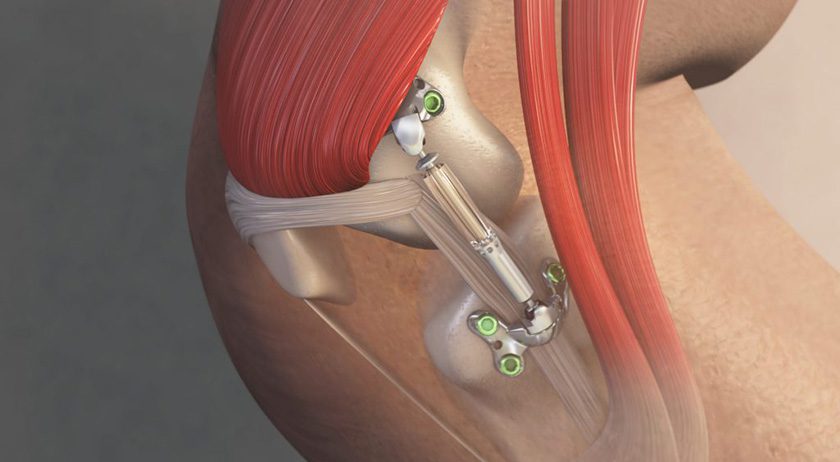
Moximed announced treatment of the first patients in a new randomized controlled trial (RCT) evaluating the MISHA Knee System.
MOTION RCT is a prospective, multicenter study comparing the benefits in subjects with medial knee osteoarthritis who are treated with either the MISHA Knee or with non-surgical treatment. The non-surgical arm features treatments supported by the American Academy of Orthopaedic Surgeons Clinical Practice Guidelines including weight loss, physical conditioning, physical therapy, assist devices, intra-articular injections and prescription or nonprescription medications.
Study Highlights
- ≥ 100 Subjects will be randomized
- ~10 Sites within the United States with patients currently enrolled
- WOMAC Pain improvement is the primary endpoint
MISHA was FDA cleared in 2023, is in commercial use and has shown to be superior to high tibial osteotomy at two years post-op. Five-year data from the pivotal FDA trial is slated for publication by the end of 2025.
“Knee OA patients across the country are looking for an effective and less invasive treatment option without activity restrictions,” said Christopher Gleason, President and Chief Executive Officer, Moximed. “The growing commercial adoption of the MISHA Knee System reflects the confidence that physicians and patients have in this breakthrough treatment. We are honored to partner with some of the most prestigious orthopaedic institutions in the United States to continue to advance the evidence around the MISHA Knee System.”
Source: Moximed
Moximed announced treatment of the first patients in a new randomized controlled trial (RCT) evaluating the MISHA Knee System.
MOTION RCT is a prospective, multicenter study comparing the benefits in subjects with medial knee osteoarthritis who are treated with either the MISHA Knee or with non-surgical treatment. The non-surgical arm features...
Moximed announced treatment of the first patients in a new randomized controlled trial (RCT) evaluating the MISHA Knee System.
MOTION RCT is a prospective, multicenter study comparing the benefits in subjects with medial knee osteoarthritis who are treated with either the MISHA Knee or with non-surgical treatment. The non-surgical arm features treatments supported by the American Academy of Orthopaedic Surgeons Clinical Practice Guidelines including weight loss, physical conditioning, physical therapy, assist devices, intra-articular injections and prescription or nonprescription medications.
Study Highlights
- ≥ 100 Subjects will be randomized
- ~10 Sites within the United States with patients currently enrolled
- WOMAC Pain improvement is the primary endpoint
MISHA was FDA cleared in 2023, is in commercial use and has shown to be superior to high tibial osteotomy at two years post-op. Five-year data from the pivotal FDA trial is slated for publication by the end of 2025.
“Knee OA patients across the country are looking for an effective and less invasive treatment option without activity restrictions,” said Christopher Gleason, President and Chief Executive Officer, Moximed. “The growing commercial adoption of the MISHA Knee System reflects the confidence that physicians and patients have in this breakthrough treatment. We are honored to partner with some of the most prestigious orthopaedic institutions in the United States to continue to advance the evidence around the MISHA Knee System.”
Source: Moximed

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.