Copy to clipboard
Copy to clipboard 
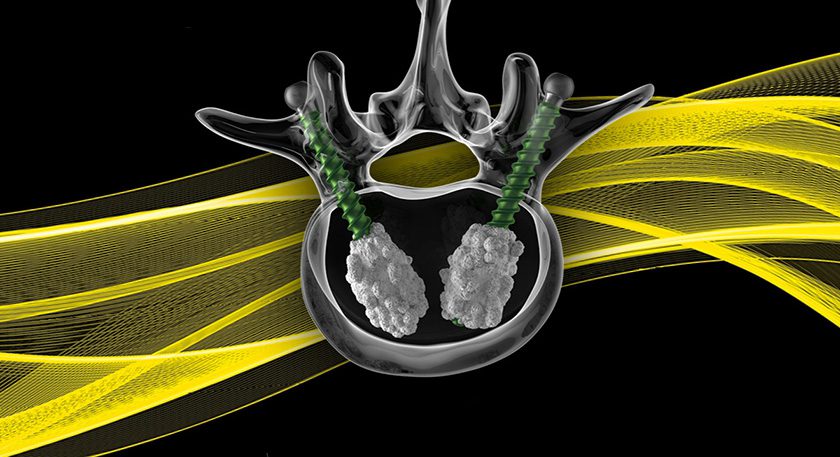
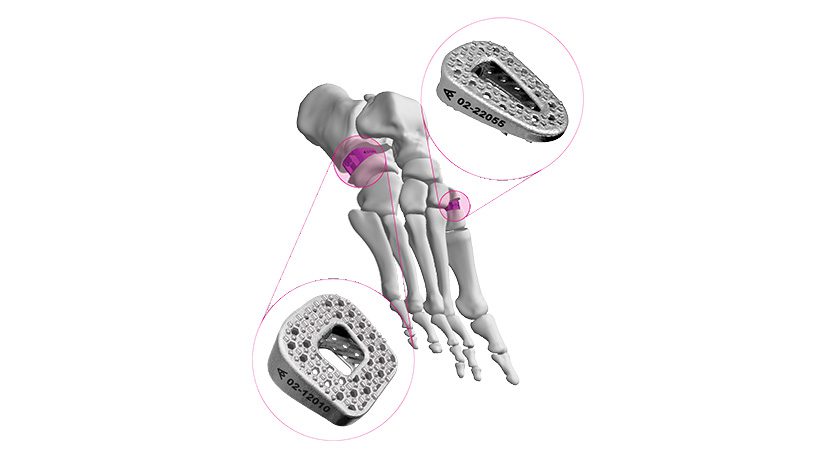
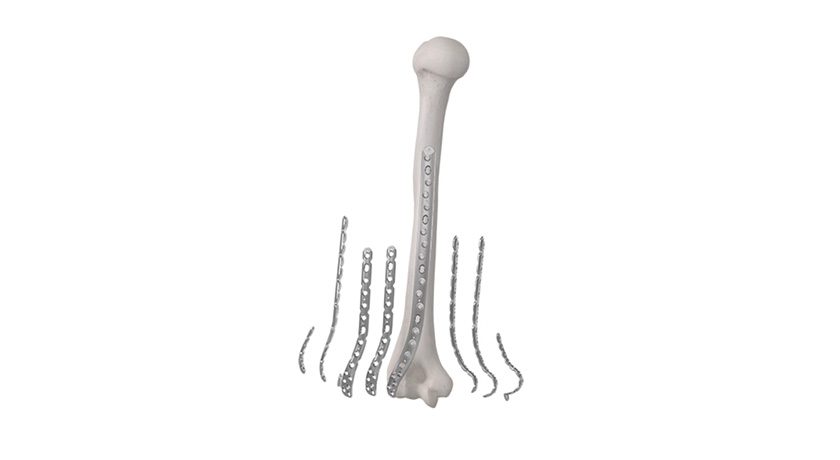
Elute received FDA 510(k) clearance to market BonVie+, a novel bone void filler implant.
BonVie+ is designed for controlled resorption and replacement by new bone.
“This new era in resorbable bone graft fillers represents over a decade of innovation, equipping clinicians with unparalleled consistency in bone restoration and predictable clinical results,” said Ashok Khandkar, CEO of Elute.
Source: Elute, Inc.
Elute received FDA 510(k) clearance to market BonVie+, a novel bone void filler implant.
BonVie+ is designed for controlled resorption and replacement by new bone.
"This new era in resorbable bone graft fillers represents over a decade of innovation, equipping clinicians with unparalleled...
Elute received FDA 510(k) clearance to market BonVie+, a novel bone void filler implant.
BonVie+ is designed for controlled resorption and replacement by new bone.
“This new era in resorbable bone graft fillers represents over a decade of innovation, equipping clinicians with unparalleled consistency in bone restoration and predictable clinical results,” said Ashok Khandkar, CEO of Elute.
Source: Elute, Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.