
 Copy to clipboard
Copy to clipboard 
CTL Medical received FDA 510(k) clearance to support the MATISSE™ Ti-PEEK ACIF cage, featuring TiCro™ surface technology.
The first version of MATISSE received 510(k) clearance in 2012, under Accel Spine. CTL acquired Accel in early 2016 and gained a next FDA clearance for MATISSE in mid-2017.
Updates to MATISSE include three material options. As with previous models, MATISSE is available in a variety of sizes and profiles. Like the original titanium-based version, the Ti-PEEK interbody is designed to support a 200% greater endplate contact surface area. The device is indicated for use with supplemental fixation, such as the company’s VAN GOGH™ Anterior cervical plate.
Source: CTL Medical Corporation
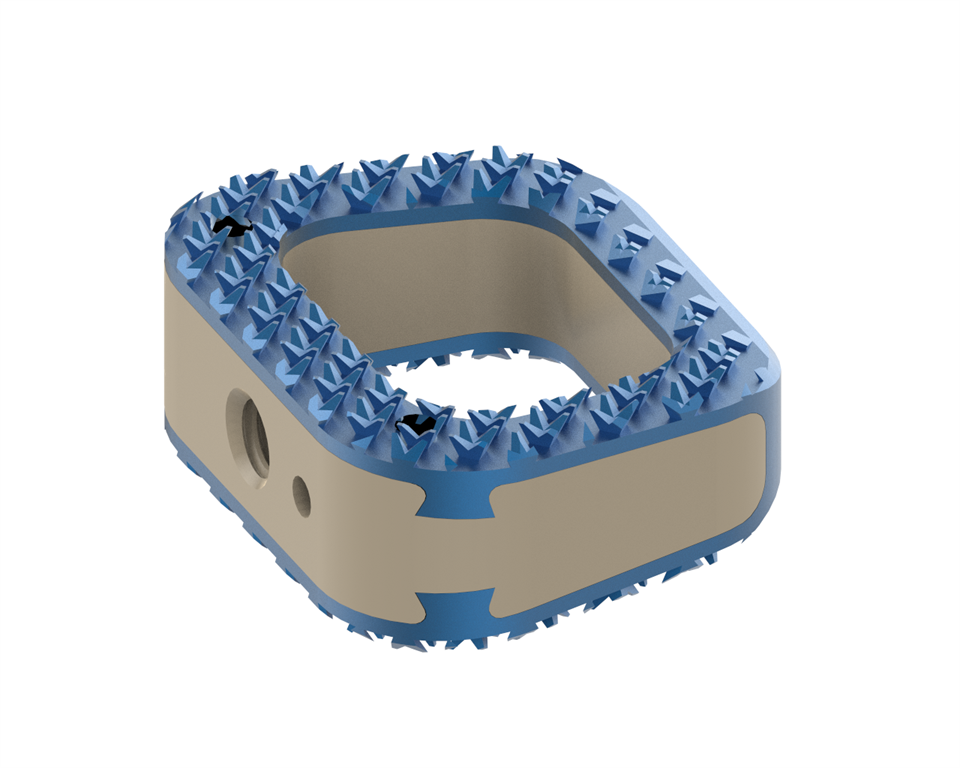
Image courtesy of CTL Medical
CTL Medical received FDA 510(k) clearance to support the MATISSE™ Ti-PEEK ACIF cage, featuring TiCro™ surface technology.
The first version of MATISSE received 510(k) clearance in 2012, under Accel Spine. CTL acquired Accel in early 2016 and gained a next FDA clearance for MATISSE in mid-2017.
Updates to MATISSE include three material...
CTL Medical received FDA 510(k) clearance to support the MATISSE™ Ti-PEEK ACIF cage, featuring TiCro™ surface technology.
The first version of MATISSE received 510(k) clearance in 2012, under Accel Spine. CTL acquired Accel in early 2016 and gained a next FDA clearance for MATISSE in mid-2017.
Updates to MATISSE include three material options. As with previous models, MATISSE is available in a variety of sizes and profiles. Like the original titanium-based version, the Ti-PEEK interbody is designed to support a 200% greater endplate contact surface area. The device is indicated for use with supplemental fixation, such as the company’s VAN GOGH™ Anterior cervical plate.
Source: CTL Medical Corporation

Image courtesy of CTL Medical

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







