
 Copy to clipboard
Copy to clipboard 
Stryker Spine received FDA 510(k) clearance to market Tritanium® TL, a 3D-printed curved posterior lumbar interbody fusion cage. The device will launch in 2Q18.
The hollow implant comprises solid and porous structures that are simultaneously built with AMagine™, Stryker’s proprietary additive manufacturing method. Tritanium highly-porous titanium material is designed to create a favorable environment for osteoblast attachment, and may be able to retain fluid unlike traditional titanium.
Tritanium TL joins other products in Stryker’s 3D-printed spine portfolio, the Tritanium PL Posterior Lumbar Cage and the Tritanium C Anterior Cervical Cage.
Sources: Stryker; ORTHOWORLD Inc.
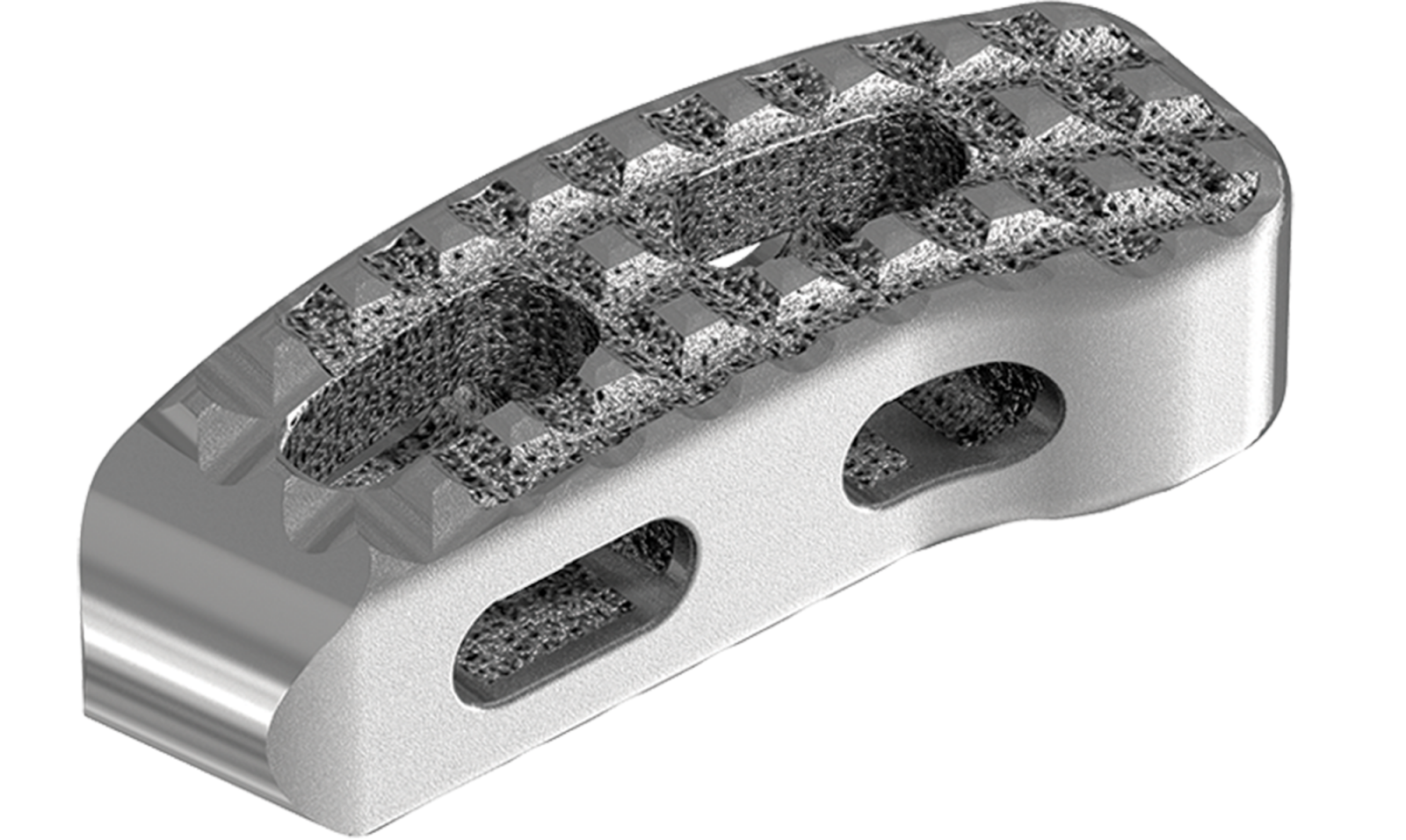
Stryker Spine received FDA 510(k) clearance to market Tritanium® TL, a 3D-printed curved posterior lumbar interbody fusion cage. The device will launch in 2Q18.
The hollow implant comprises solid and porous structures that are simultaneously built with AMagine™, Stryker’s proprietary additive manufacturing method. Tritanium highly-porous...
Stryker Spine received FDA 510(k) clearance to market Tritanium® TL, a 3D-printed curved posterior lumbar interbody fusion cage. The device will launch in 2Q18.
The hollow implant comprises solid and porous structures that are simultaneously built with AMagine™, Stryker’s proprietary additive manufacturing method. Tritanium highly-porous titanium material is designed to create a favorable environment for osteoblast attachment, and may be able to retain fluid unlike traditional titanium.
Tritanium TL joins other products in Stryker’s 3D-printed spine portfolio, the Tritanium PL Posterior Lumbar Cage and the Tritanium C Anterior Cervical Cage.
Sources: Stryker; ORTHOWORLD Inc.


You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







