Copy to clipboard
Copy to clipboard 
Theradaptive closed a $26 million Series A funding round to advance development of its targeted regenerative therapeutics.
The funds will enable the company to continue its work to meet regulatory requirements and scale up Good Manufacturing Practices in preparation for first-in-human clinical trials later this year. Investment will also support the expansion of the Theradaptive platform beyond orthopedics to additional indications such as oncology and dental. The round brings Theradaptive’s total funding to over $50 million.
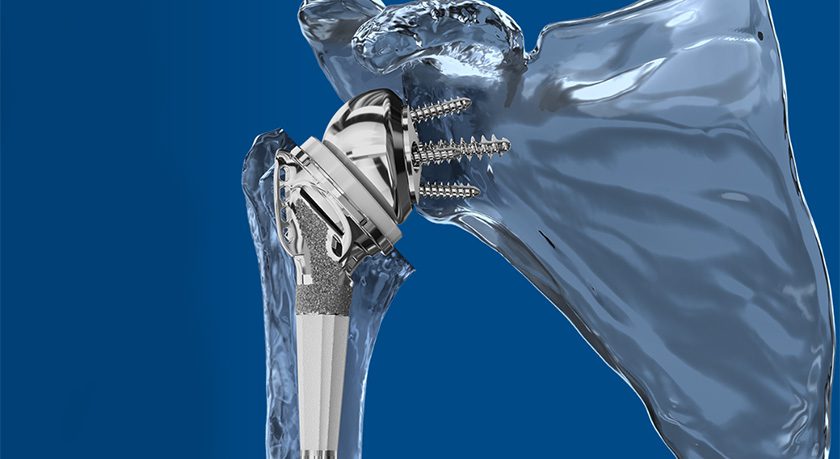
Theradaptive’s proprietary protein-engineering platform produces targeted therapeutics that can be used to coat implants, devices and injectable carriers to achieve hyper-local delivery over long time periods exceeding weeks to months.
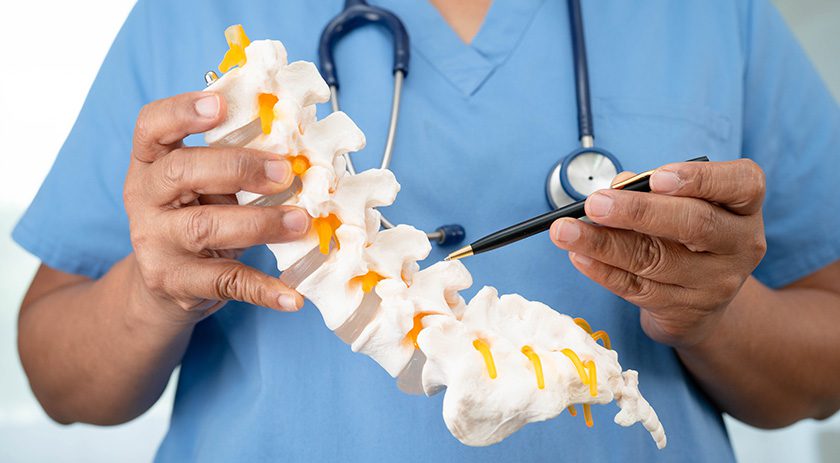
With this technology, FDA has granted an unprecedented three breakthrough medical device designations to Theradaptive for its various spinal indications.
Source: Theradaptive
Theradaptive closed a $26 million Series A funding round to advance development of its targeted regenerative therapeutics.
The funds will enable the company to continue its work to meet regulatory requirements and scale up Good Manufacturing Practices in preparation for first-in-human clinical trials later this year. Investment will also...
Theradaptive closed a $26 million Series A funding round to advance development of its targeted regenerative therapeutics.
The funds will enable the company to continue its work to meet regulatory requirements and scale up Good Manufacturing Practices in preparation for first-in-human clinical trials later this year. Investment will also support the expansion of the Theradaptive platform beyond orthopedics to additional indications such as oncology and dental. The round brings Theradaptive’s total funding to over $50 million.
Theradaptive’s proprietary protein-engineering platform produces targeted therapeutics that can be used to coat implants, devices and injectable carriers to achieve hyper-local delivery over long time periods exceeding weeks to months.
With this technology, FDA has granted an unprecedented three breakthrough medical device designations to Theradaptive for its various spinal indications.
Source: Theradaptive

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.