Copy to clipboard
Copy to clipboard 
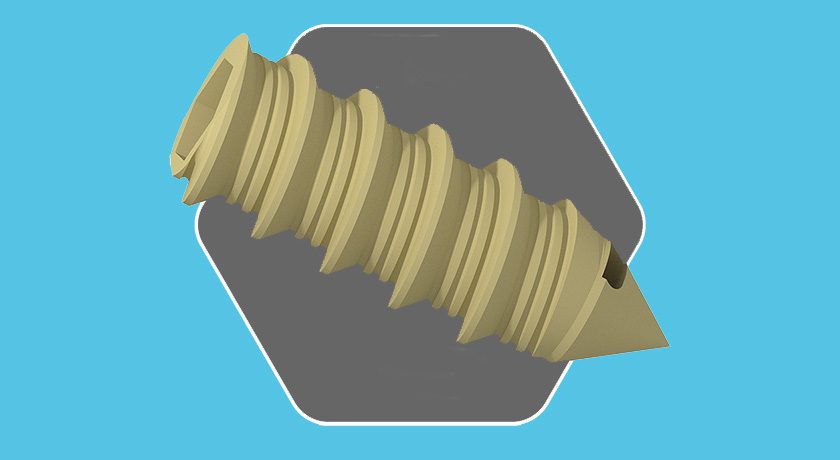
Medtronic is recalling all lot numbers of KYPHON® Directional Bone Void Filler (Product # F04C) due to a misalignment issue that may cause injected cement to be placed in an unintended direction.
The recall is ranked as Class II, with ~17,650 units distributed worldwide.
Possible risks include cement extravasation into the spinal canal, with potential for paralysis or nerve injury with risk of pulmonary embolism or cardiac arrest. The company has received two reports of misalignment, and no associated patient injuries are reported to have resulted from this issue.
Sources: MHRA Field Safety Notice, MHRA.gov; Recall Notice, FDA.gov
Medtronic is recalling all lot numbers of KYPHON® Directional Bone Void Filler (Product # F04C) due to a misalignment issue that may cause injected cement to be placed in an unintended direction.
The recall is ranked as Class II, with 7,650 units distributed worldwide.
Possible risks include cement extravasation into the spinal canal,...
Medtronic is recalling all lot numbers of KYPHON® Directional Bone Void Filler (Product # F04C) due to a misalignment issue that may cause injected cement to be placed in an unintended direction.
The recall is ranked as Class II, with ~17,650 units distributed worldwide.
Possible risks include cement extravasation into the spinal canal, with potential for paralysis or nerve injury with risk of pulmonary embolism or cardiac arrest. The company has received two reports of misalignment, and no associated patient injuries are reported to have resulted from this issue.
Sources: MHRA Field Safety Notice, MHRA.gov; Recall Notice, FDA.gov

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.