Copy to clipboard
Copy to clipboard 
DJO Global announced FDA 510(k) clearance for the Exprt® Revision Hip, to be priced up to 70% lower than comparable revision hip devices.
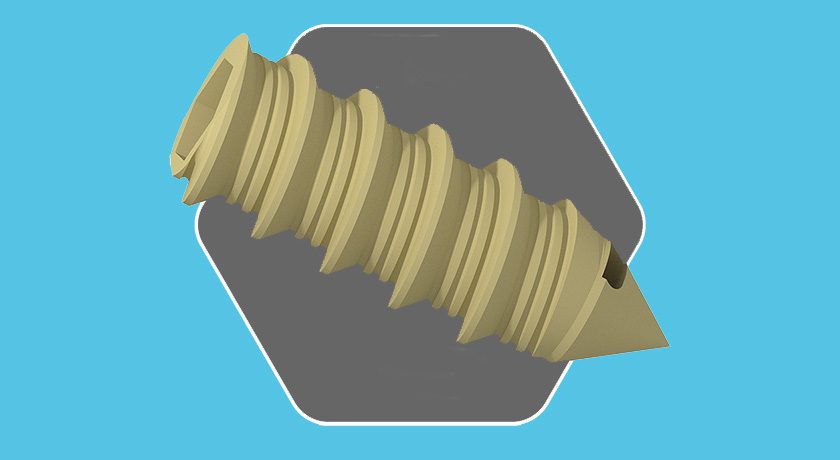
The full-line modular femoral stem was inspired by the clinical success of Wagner-style implants, and offers an anatomical design. Further, the two-tray system represents an 80% to 90% reduction in instruments vs. competitive systems.
The company’s Exprt™ Precision revision knee launched in 2015.
DJO Global is offering a preview of the Exprt Revision Hip at the 2017 AAOS meeting in Booth #1733.
Sources: DJO Global; ORTHOWORLD Inc.
DJO Global announced FDA 510(k) clearance for the Exprt® Revision Hip, to be priced up to 70% lower than comparable revision hip devices.
The full-line modular femoral stem was inspired by the clinical success of Wagner-style implants, and offers an anatomical design. Further, the two-tray system represents an 80% to 90% reduction in...
DJO Global announced FDA 510(k) clearance for the Exprt® Revision Hip, to be priced up to 70% lower than comparable revision hip devices.
The full-line modular femoral stem was inspired by the clinical success of Wagner-style implants, and offers an anatomical design. Further, the two-tray system represents an 80% to 90% reduction in instruments vs. competitive systems.
The company’s Exprt™ Precision revision knee launched in 2015.
DJO Global is offering a preview of the Exprt Revision Hip at the 2017 AAOS meeting in Booth #1733.
Sources: DJO Global; ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.