Copy to clipboard
Copy to clipboard 
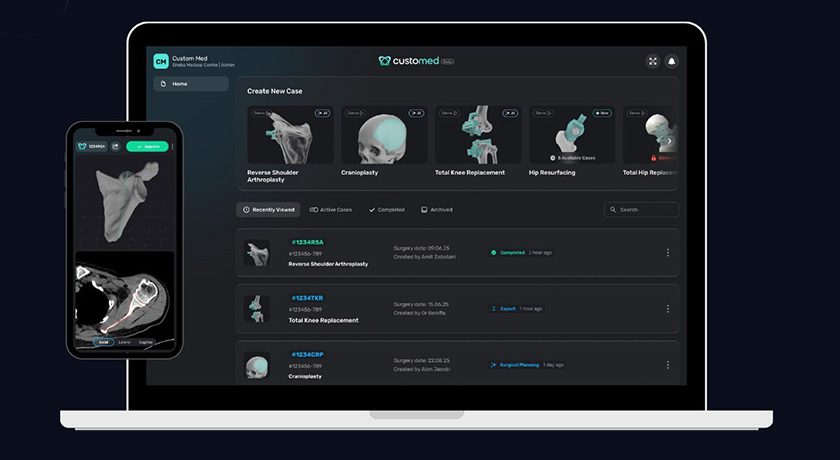
Spineart secured a €30MM (~US $33MM) investment. Proceeds will support sales, geographical expansion in markets such as the U.S. and Europe and product development.
Funds were provided by Gimv, an investment company, and Health and Care Fund.
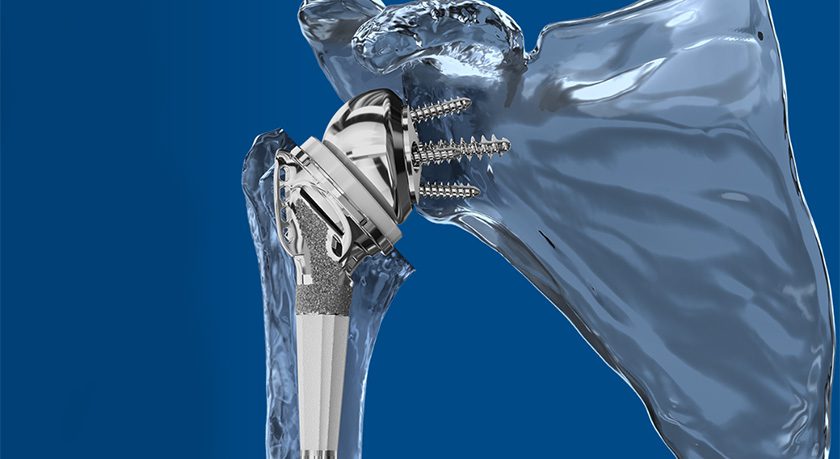
Spineart has secured numerous FDA 510(k) clearances in the U.S. since 2008, and was recently granted CE Mark Approval for its JULIET®Ti lumbar interbody systems featuring TI-LIFE, a micro-porous scaffold designed to mimic trabecular bone.
Sources: Spineart; FDA.gov; ORTHOWORLD Inc.
Spineart secured a €30MM (~US $33MM) investment. Proceeds will support sales, geographical expansion in markets such as the U.S. and Europe and product development.
Funds were provided by Gimv, an investment company, and Health and Care Fund.
Spineart has secured numerous FDA 510(k) clearances in the U.S. since 2008, and was recently...
Spineart secured a €30MM (~US $33MM) investment. Proceeds will support sales, geographical expansion in markets such as the U.S. and Europe and product development.
Funds were provided by Gimv, an investment company, and Health and Care Fund.
Spineart has secured numerous FDA 510(k) clearances in the U.S. since 2008, and was recently granted CE Mark Approval for its JULIET®Ti lumbar interbody systems featuring TI-LIFE, a micro-porous scaffold designed to mimic trabecular bone.
Sources: Spineart; FDA.gov; ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.