Copy to clipboard
Copy to clipboard 
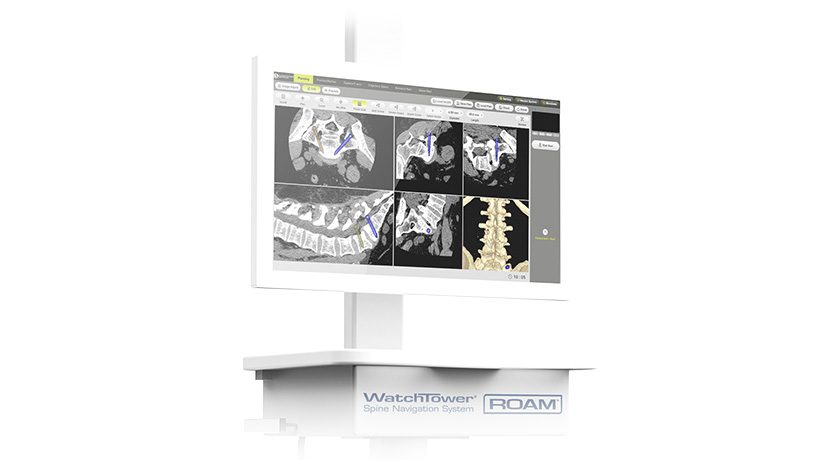
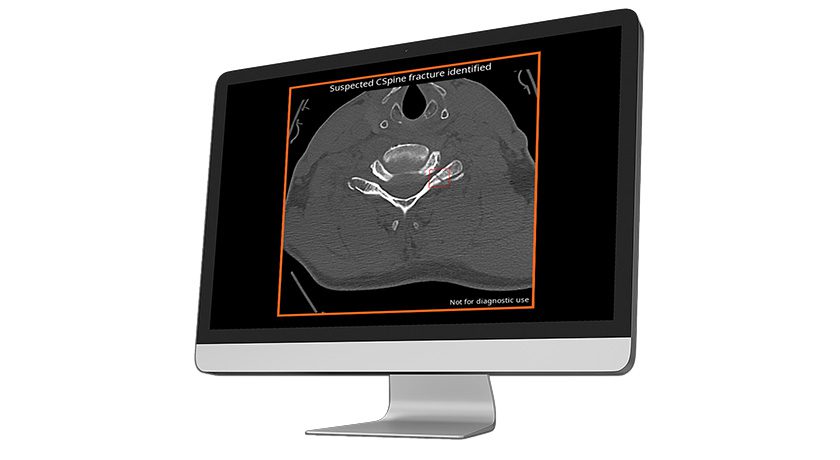
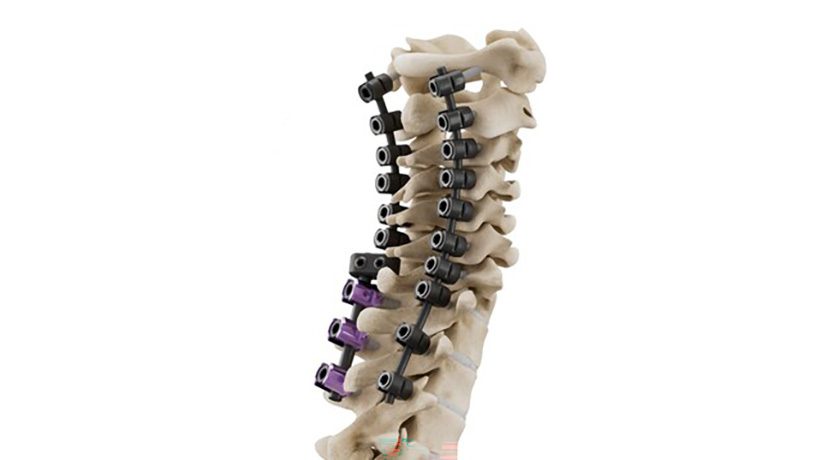
SpineGuard received FDA 510(k) clearance to market the PediGuard Threaded DSG™ device for Dynamic Surgical Guidance. The system supports open or minimally invasive approaches for pedicle screw insertion.
The system launched in the EU and Asia earlier this year, following approval under the CE Mark.
Sources: SpineGuard, ORTHOWORLD Inc.
SpineGuard received FDA 510(k) clearance to market the PediGuard Threaded DSG™ device for Dynamic Surgical Guidance. The system supports open or minimally invasive approaches for pedicle screw insertion.
The system launched in the EU and Asia earlier this...
SpineGuard received FDA 510(k) clearance to market the PediGuard Threaded DSG™ device for Dynamic Surgical Guidance. The system supports open or minimally invasive approaches for pedicle screw insertion.
The system launched in the EU and Asia earlier this year, following approval under the CE Mark.
Sources: SpineGuard, ORTHOWORLD Inc.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.