Copy to clipboard
Copy to clipboard 
ConforMIS (CFMS) withdrew its FDA 510(k) application for the iTotal Hip device, and plans to submit a new application in 2H16.
CFMS submitted the application for the customized total hip replacement, as planned, in 2015. In light of an inability to address FDA’s review questions within an allowed review timeframe, CFMS elected to withdraw the application. The company will address FDA’s questions and conduct any associated testing before submission of a new application for 510(k) clearance of the iTotal Hip.
Source: ConforMIS, Inc.
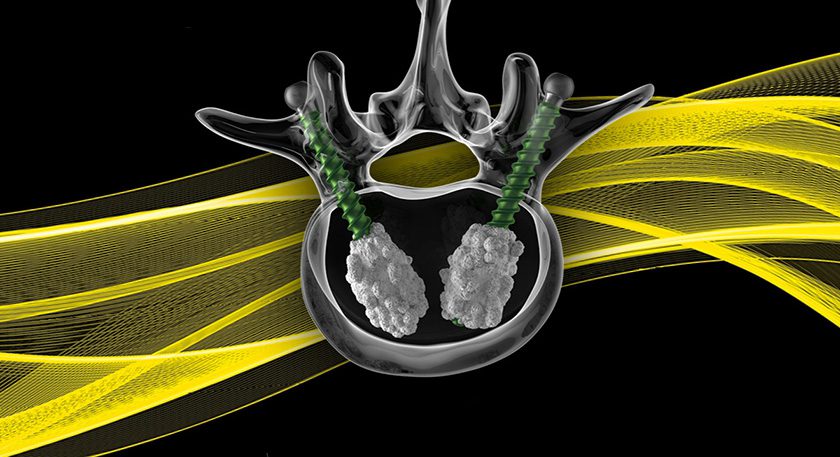
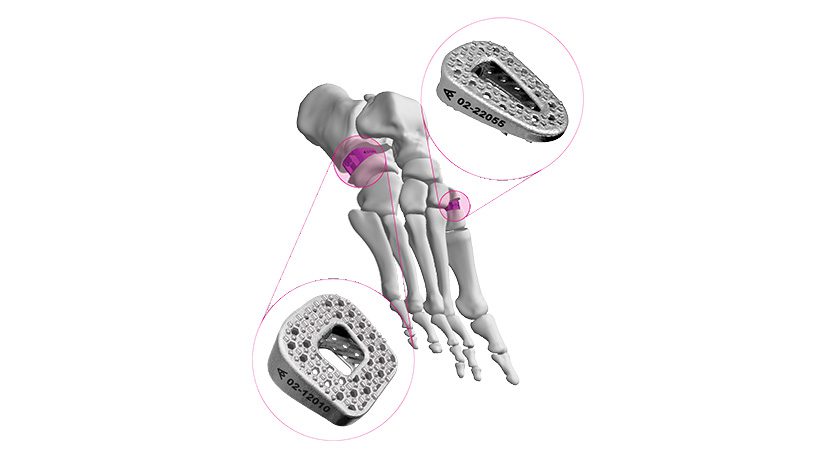
The company’s proprietary iFit Image-to-Implant® technology platform is presently used to develop and manufacture knee replacement implants that are individually-sized and shaped to a patient’s anatomy.
CFMS claims that its ability to provide long-term outcomes and economic efficiency data for its custom implants and technology give it an ideal edge in the era of bundled payments, part of Medicare’s Comprehensive Care for Joint Replacement/CJR program.
ConforMIS (CFMS) withdrew its FDA 510(k) application for the iTotal Hip device, and plans to submit a new application in 2H16.
CFMS submitted the application for the customized total hip replacement, as planned, in 2015. In light of an inability to address FDA's review questions within an allowed review timeframe, CFMS elected to withdraw the...
ConforMIS (CFMS) withdrew its FDA 510(k) application for the iTotal Hip device, and plans to submit a new application in 2H16.
CFMS submitted the application for the customized total hip replacement, as planned, in 2015. In light of an inability to address FDA’s review questions within an allowed review timeframe, CFMS elected to withdraw the application. The company will address FDA’s questions and conduct any associated testing before submission of a new application for 510(k) clearance of the iTotal Hip.
Source: ConforMIS, Inc.
The company’s proprietary iFit Image-to-Implant® technology platform is presently used to develop and manufacture knee replacement implants that are individually-sized and shaped to a patient’s anatomy.
CFMS claims that its ability to provide long-term outcomes and economic efficiency data for its custom implants and technology give it an ideal edge in the era of bundled payments, part of Medicare’s Comprehensive Care for Joint Replacement/CJR program.

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.