ORTHOFLASH®
TigerShark Lateral uses the proprietary BioBond porous trabecular structure to create the new lateral interbody system.
Introduction of the pre-op planning and modeling system completes the CORIOGRAPH Services portfolio, which includes solutions for knee and hip arthroplasty.
Bioretec’s RemeOs screw is the first and only osteopromotive absorbable metal implant for orthopedic use in the U.S.
September 26, 2025
Julie A. Vetalice
September 22, 2025
,
Julie A. Vetalice
September 19, 2025
,
Julie A. Vetalice
TigerShark Lateral uses the proprietary BioBond porous trabecular structure to create the new lateral interbody system.
October 6, 2025
,
Introduction of the pre-op planning and modeling system completes the CORIOGRAPH Services portfolio, which includes solutions for knee and hip arthroplasty.
October 2, 2025
,
Bioretec’s RemeOs screw is the first and only osteopromotive absorbable metal implant for orthopedic use in the U.S.
October 1, 2025
,
The iTaperloc Complete and iG7 Hip System with Iodine Technology inhibits bacterial adhesion and prevents biofilm formation on the implant surface to address PJI.
September 26, 2025
,
With a tiny, high-quality camera on the tip of a needle-like device, the scope requires only a small incision yielding minimal tissue disruption and a smaller scar.
September 23, 2025
,
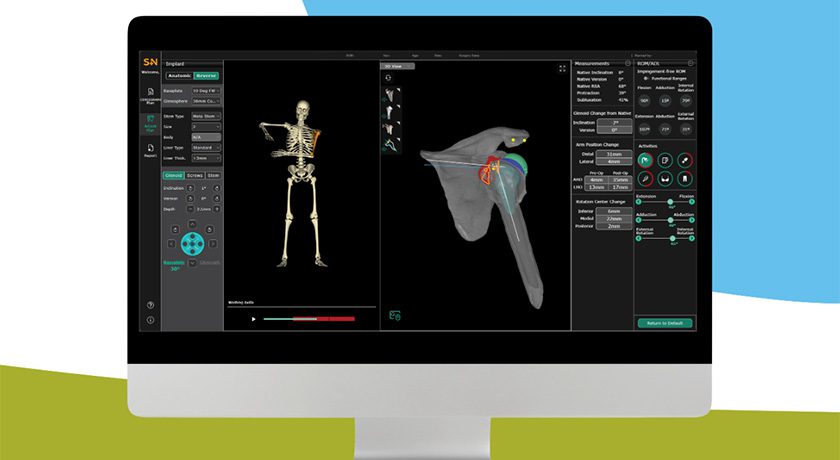
Shoulder Innovations’ InSet 70 addresses osteoarthritis and avascular necrosis, rheumatoid and traumatic arthritis, and correction of functional deformity.
September 22, 2025
,
By combining novel low-dose fluoroscopic hardware with an AI-enabled imaging solution, ODI addresses the limitations of 2D x-ray and CT systems.
September 19, 2025
,
Spine Mixed Reality Navigation combines the precision of the company’s signature optical navigation system with advanced mixed reality.
September 18, 2025
,
DEXA-L is a first-of-its-kind standalone device for use in anterior lumbar interbody fusion procedures.
September 18, 2025
,
The device is designed to work in tandem with Ruthless Spine’s De Novo cleared RJB intraoperative angle measurement instrument.
September 18, 2025
,
The VCFix Spinal System is a vertebral augmentation system designed to treat a broad spectrum of vertebral compression fractures.
September 17, 2025
,
Medartis’ TOUCH implant addresses a clinical need in hand surgery, as the CMC 1 joint is among the most commonly affected by osteoarthritis.
September 17, 2025
,
HyperFlex Bunion Correction is designed for early intervention, aiming to reduce pain, accelerate recovery and lower complication risk.
September 16, 2025
,
Cleared by FDA as the All-Suture Dual Anchor System, RapidFix is intended for fixation of soft tissue to bone in a variety of orthopedic procedures.
September 16, 2025
,
At follow-up after 11 months, treatment success, defined as complete regression of infection without recurrence, was observed in 96.1% of patients.