
 Copy to clipboard
Copy to clipboard 
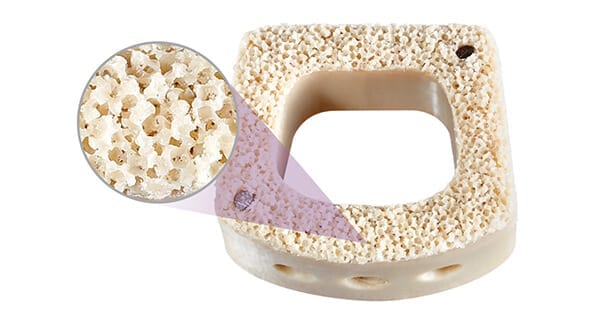
NuVasive commenced commercial launch of Cohere® XLIF®, the first Porous PEEK™ interbody for use in eXtreme Lateral Interbody Fusion and X360™ surgeries.
The company also launched the Cohere XLIF AMS plate, a low profile anti-migration plate that allows for up to two points of fixation.
Studies of the Porous PEEK in Cohere XLIF in cervical spine fusion vs. smooth PEEK and structural allograft bone indicate that patients treated with Porous PEEK had significant improvements in disability index and pain scores as early as six weeks post-op, vs. those treated with the standard implants.
“NuVasive’s Cohere XLIF implant is another example of how we are combining expertise in lateral implant design with our clinically validated porous surface technology,” said Massimo Calafiore, Executive Vice President, Global Business Units at NuVasive. “These new offerings strengthen NuVasive’s AMS portfolio and equip surgeons with best-in-class technology that procedurally integrate with XLIF and X360 procedures to enable better patient outcomes in spine surgery.”
NuVasive commenced commercial launch of Cohere® XLIF®, the first Porous PEEK™ interbody for use in eXtreme Lateral Interbody Fusion and X360™ surgeries.
The company also launched the Cohere XLIF AMS plate, a low profile anti-migration plate that allows for up to two points of fixation.
Studies of the Porous PEEK in Cohere XLIF in...
NuVasive commenced commercial launch of Cohere® XLIF®, the first Porous PEEK™ interbody for use in eXtreme Lateral Interbody Fusion and X360™ surgeries.
The company also launched the Cohere XLIF AMS plate, a low profile anti-migration plate that allows for up to two points of fixation.
Studies of the Porous PEEK in Cohere XLIF in cervical spine fusion vs. smooth PEEK and structural allograft bone indicate that patients treated with Porous PEEK had significant improvements in disability index and pain scores as early as six weeks post-op, vs. those treated with the standard implants.
“NuVasive’s Cohere XLIF implant is another example of how we are combining expertise in lateral implant design with our clinically validated porous surface technology,” said Massimo Calafiore, Executive Vice President, Global Business Units at NuVasive. “These new offerings strengthen NuVasive’s AMS portfolio and equip surgeons with best-in-class technology that procedurally integrate with XLIF and X360 procedures to enable better patient outcomes in spine surgery.”

You’ve reached your limit.
We’re glad you’re finding value in our content — and we’d love for you to keep going.
Subscribe now for unlimited access to orthopedic business intelligence.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







