
 Copy to clipboard
Copy to clipboard 
4WEB Medical received FDA 510(k) clearance for its Cervical Spine Truss System-Stand Alone (CSTS-SA) interbody fusion device. 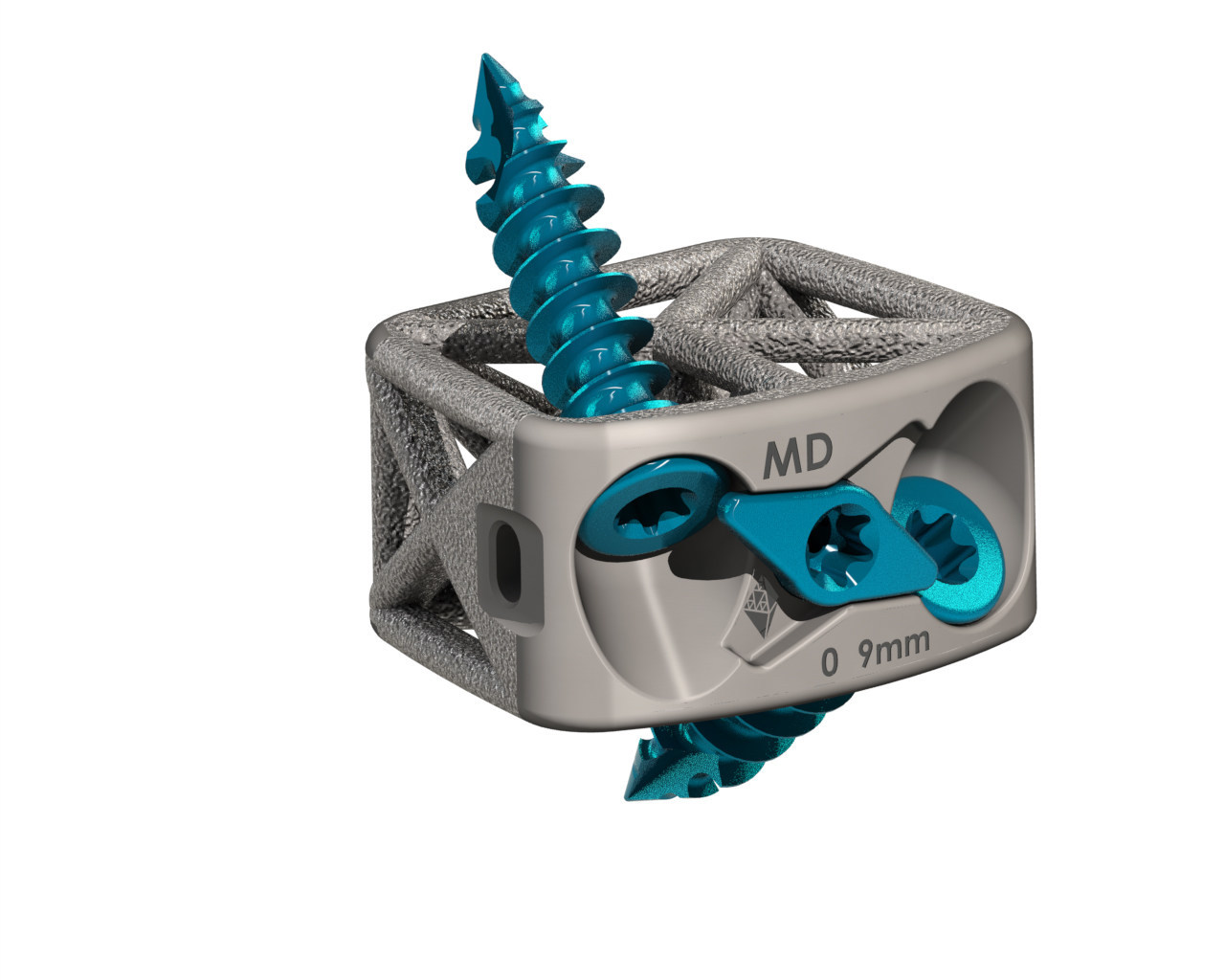
CSTS-SA incorporates 4WEB’s proprietary Truss Implant Technology. The interbody’s design allows fixation screws to be placed through the truss implant and into the adjacent vertebral bodies, creating a zero-profile stand-alone construct. The 3D-printed device also features a single-step locking mechanism.
4WEB will launch the sterile-packed CSTS-SA in multiple footprints, lordotic angles and heights. The device is expected to be available in 4Q19.
Source: 4WEB Medical
4WEB Medical received FDA 510(k) clearance for its Cervical Spine Truss System-Stand Alone (CSTS-SA) interbody fusion device.
CSTS-SA incorporates 4WEB’s proprietary Truss Implant Technology. The interbody's design allows fixation screws to be placed through the truss implant and into the adjacent vertebral bodies, creating a zero-profile...
4WEB Medical received FDA 510(k) clearance for its Cervical Spine Truss System-Stand Alone (CSTS-SA) interbody fusion device. 
CSTS-SA incorporates 4WEB’s proprietary Truss Implant Technology. The interbody’s design allows fixation screws to be placed through the truss implant and into the adjacent vertebral bodies, creating a zero-profile stand-alone construct. The 3D-printed device also features a single-step locking mechanism.
4WEB will launch the sterile-packed CSTS-SA in multiple footprints, lordotic angles and heights. The device is expected to be available in 4Q19.
Source: 4WEB Medical

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







