
 Copy to clipboard
Copy to clipboard 
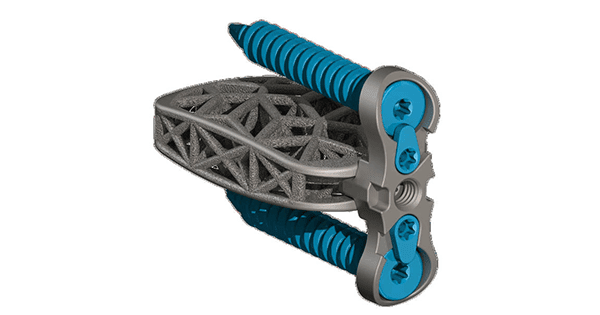
4WEB Medical was granted FDA 510(k) clearance from to market its Lumbar Plating Solution (LSTS-PS).
The new system comprises a variety of modular plating configurations to address multiple lumbar spine pathologies and approaches. The device provides an integrated and non-integrated offering with a one, two and four screw option. The plate design also features a single-step locking mechanism to prevent screw backout.
The company expects to launch its hyperlordotic lateral implant line and an Anterior-to-Psoas implant by the end of 2021.
“Our continued commitment to new product development led to revenue growth in FY 2020 driven by the launch of the Stand Alone Cervical and ALIF product lines,” said Jim Bruty, Sr. Vice President of Sales and Marketing. “The Lumbar Plating Solution will contribute significantly to 2021 top line growth by enabling surgeon customers to utilize the company’s Truss Implant Technology in more advanced lateral procedures such as anterior longitudinal ligament release techniques.”
4WEB Medical was granted FDA 510(k) clearance from to market its Lumbar Plating Solution (LSTS-PS).
The new system comprises a variety of modular plating configurations to address multiple lumbar spine pathologies and approaches. The device provides an integrated and non-integrated offering with a one, two and four screw option. The plate...
4WEB Medical was granted FDA 510(k) clearance from to market its Lumbar Plating Solution (LSTS-PS).
The new system comprises a variety of modular plating configurations to address multiple lumbar spine pathologies and approaches. The device provides an integrated and non-integrated offering with a one, two and four screw option. The plate design also features a single-step locking mechanism to prevent screw backout.
The company expects to launch its hyperlordotic lateral implant line and an Anterior-to-Psoas implant by the end of 2021.
“Our continued commitment to new product development led to revenue growth in FY 2020 driven by the launch of the Stand Alone Cervical and ALIF product lines,” said Jim Bruty, Sr. Vice President of Sales and Marketing. “The Lumbar Plating Solution will contribute significantly to 2021 top line growth by enabling surgeon customers to utilize the company’s Truss Implant Technology in more advanced lateral procedures such as anterior longitudinal ligament release techniques.”

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







