Copy to clipboard
Copy to clipboard 
3Spine, developer of a total joint replacement for the lumbar spine, raised $33 million in an oversubscribed Series C private offering. Proceeds will support Phase II clinical study of the BalancedBack Total Joint Replacement in the U.S. To date, the company has raised a total of $50.3 million from private investors.
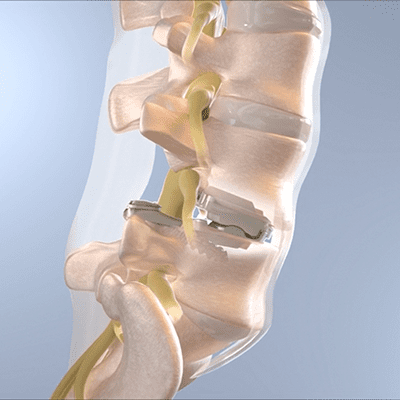
3Spine’s MOTUS device, the implant used in the BalancedBack Total Joint Replacement procedure, replaces the function of the disc and facet joints through a posterior approach. The procedure broadly addresses leg pain, back pain and spinal instability, while correcting posture and restoring freedom of movement by reconstructing the functional spinal unit. Per 3Spine, the technology is the only 1:1 replacement for lumbar spinal fusion, with an estimated annual U.S. addressable market size of over $5 billion.
MOTUS was designated a Breakthrough Device by FDA in 2020 and in early 2022, the American Medical Association assigned a CPT Code to track outpatient utilization of lumbar total joint replacement as a new procedure. 3Spine is currently enrolling a 150-patient prospective Real-World Evidence study at 16 clinical sites across 11 states. The company is additionally enrolling three prospective longitudinal cohorts outside of the U.S. for single-level disease, two-level disease and joint replacement adjacent to existing fusion constructs. 3Spine anticipates Investigative Device Exemption approval in early 2Q22.
Source: 3Spine
3Spine, developer of a total joint replacement for the lumbar spine, raised $33 million in an oversubscribed Series C private offering. Proceeds will support Phase II clinical study of the BalancedBack Total Joint Replacement in the U.S. To date, the company has raised a total of $50.3 million from private investors.
3Spine's MOTUS device, the...
3Spine, developer of a total joint replacement for the lumbar spine, raised $33 million in an oversubscribed Series C private offering. Proceeds will support Phase II clinical study of the BalancedBack Total Joint Replacement in the U.S. To date, the company has raised a total of $50.3 million from private investors.
3Spine’s MOTUS device, the implant used in the BalancedBack Total Joint Replacement procedure, replaces the function of the disc and facet joints through a posterior approach. The procedure broadly addresses leg pain, back pain and spinal instability, while correcting posture and restoring freedom of movement by reconstructing the functional spinal unit. Per 3Spine, the technology is the only 1:1 replacement for lumbar spinal fusion, with an estimated annual U.S. addressable market size of over $5 billion.
MOTUS was designated a Breakthrough Device by FDA in 2020 and in early 2022, the American Medical Association assigned a CPT Code to track outpatient utilization of lumbar total joint replacement as a new procedure. 3Spine is currently enrolling a 150-patient prospective Real-World Evidence study at 16 clinical sites across 11 states. The company is additionally enrolling three prospective longitudinal cohorts outside of the U.S. for single-level disease, two-level disease and joint replacement adjacent to existing fusion constructs. 3Spine anticipates Investigative Device Exemption approval in early 2Q22.
Source: 3Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.