
 Copy to clipboard
Copy to clipboard 
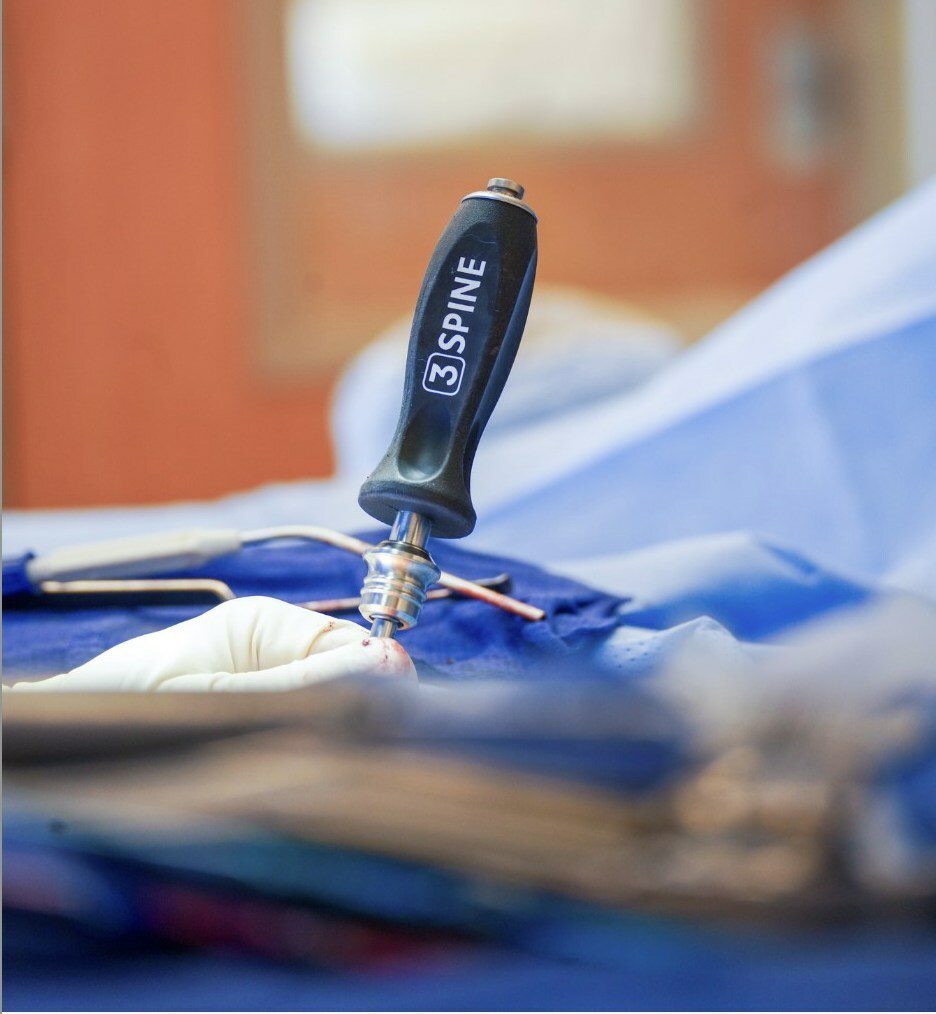
3Spine achieved U.S. clinical trial enrollment, with 151 completed MOTUS lumbar total joint replacement surgeries and 174 real-world posterior lumbar fusions as of December 2023. The company enrolled a larger fusion cohort in an adaptive statistical design to improve propensity score matching between data sets. The trials involved patients experiencing chronic leg and back pain ranging in age from 23 to 79 across 14 U.S. states. 3Spine plans to seek FDA approval through the Premarket Approval pathway.
3Spine’s MOTUS device is a breakthrough medical technology indicated for the biomechanical reconstruction and stabilization of a spinal motion segment following decompression at one lumbar level from L1/L2 to L5/S1. MOTUS replaces the function of the disc and facet joints through a posterior approach, where the dual bearings resist rotation and shear much like the two native facet joints, while carrying compressive axial loads like the disc. The technology is similar to other modern orthopedic joint replacements, utilizing highly cross-linked antioxidant vitamin E polyethylene, compression molded into a CoCr, titanium plasma sprayed substrate.
MOTUS has been in development for over two decades. In 2020, FDA designated MOTUS as a Breakthrough Device.
Source: 3Spine
3Spine achieved U.S. clinical trial enrollment, with 151 completed MOTUS lumbar total joint replacement surgeries and 174 real-world posterior lumbar fusions as of December 2023. The company enrolled a larger fusion cohort in an adaptive statistical design to improve propensity score matching between data sets. The trials involved patients...
3Spine achieved U.S. clinical trial enrollment, with 151 completed MOTUS lumbar total joint replacement surgeries and 174 real-world posterior lumbar fusions as of December 2023. The company enrolled a larger fusion cohort in an adaptive statistical design to improve propensity score matching between data sets. The trials involved patients experiencing chronic leg and back pain ranging in age from 23 to 79 across 14 U.S. states. 3Spine plans to seek FDA approval through the Premarket Approval pathway.
3Spine’s MOTUS device is a breakthrough medical technology indicated for the biomechanical reconstruction and stabilization of a spinal motion segment following decompression at one lumbar level from L1/L2 to L5/S1. MOTUS replaces the function of the disc and facet joints through a posterior approach, where the dual bearings resist rotation and shear much like the two native facet joints, while carrying compressive axial loads like the disc. The technology is similar to other modern orthopedic joint replacements, utilizing highly cross-linked antioxidant vitamin E polyethylene, compression molded into a CoCr, titanium plasma sprayed substrate.
MOTUS has been in development for over two decades. In 2020, FDA designated MOTUS as a Breakthrough Device.
Source: 3Spine

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







