

 Copy to clipboard
Copy to clipboard 
Companies across all orthopedic market segments with products in development or recently cleared continue to secure funding to fuel commercialization efforts. We recap 18 funding announcements that took place since June 2021. Many of the companies are familiar names with previously-closed funding rounds.
Joint Replacement
Catalyst OrthoScience raised $12.3 million in an oversubscribed Series D financing round.
Withing 2021, the shoulder replacement manufacturer has rebranded its product portfolio with the Archer family name, received FDA 510(k) clearance and began a limited launch for the Archer R1 Reverse Shoulder System, as well as introduced its Archer 3D Targeting imaging software for its current product offerings.
Additionally, the Archer CSR Total Shoulder System, Catalyst’s stemless, ellipsoid anatomic system, has continued rapid adoption and growth. Archer shoulder systems have been used in more than 3,000 surgeries.

Catalyst OrthoScience Archer R1 Reverse Shoulder
Garwood Medical Devices, developer of BioPrax technology to treat antibiotic-resistant bacterial biofilm infections associated with metallic orthopedic implants, completed a Series C round that closed at $4 million, as projected.
The company has now raised $11.4 million, including $3.8 million in the Series B round, which closed in late 2019. The company raised an initial $3.6 million in a Series A financing round, which closed in 2016. Funds raised will continue to support preclinical testing.
BioPrax is under investigation to study the elimination of biofilm infections on prosthetic knee implants during early intervention procedures, when used alongside the current standard of care.
BioPrax was accepted into FDA’s Breakthrough Device Program in 2019. The product is expected to be on the market in 2025.

Garwood Medical BioPrax Technology
MatOrtho received an investment of £6.7 million (~USD $9.4 million) to support its international growth and product development. The company produces knee, hip and finger joint replacement systems.
The funding will enable MatOrtho to accelerate international expansion into existing and new markets and will support research and development as the company continues to develop the next generation of implant technology.
Onkos Surgical raised $15 million in Series C funding.
The company markets implants and instruments that meet the needs of musculoskeletal tumor patients, with a focus on clinical challenges of implant loosening, soft tissue failures and infection.
Proceeds will drive revenue growth by accelerating the company’s research and development pipeline, expanding its commercial footprint and evolving manufacturing capabilities.
Spine
Empirical Spine closed a Series B financing of $10 million. Funds will advance the commercialization of the LimiFlex Paraspinous Tension Band, including completion of the ongoing pivotal trial and submitting all components of a premarket approval filing to FDA.
LimiFlex was developed to offer a minimally invasive means to stabilize the spine yet preserve motion.
The prospective, multicenter, controlled clinical trial is comparing LimiFlex Paraspinous Tension to transforaminal lumbar interbody fusion in the treatment of degenerative spondylolisthesis and lumbar spinal stenosis.
MiRus completed a highly oversubscribed $65 million funding round for growth of its spine and extremity portfolios and development of structural heart disease solutions using its proprietary Rhenium-based alloys (MoRe).
Implants made from Rhenium-based superalloys are reported to have superior mechanical strength, fatigue resistance and dramatically better biological performance vs. titanium, cobalt and nickel-based alloys used in medical implants.
ReVivo Medical completed its most recent equity offering.
Overall, the company has raised $2 million which will support completion of 50 surgical procedures in a clinical trial. Study participants will receive ReVivo Medical’s next-generation design anterior cervical plate and interbody cages used in anterior cervical discectomy and fusion procedures.
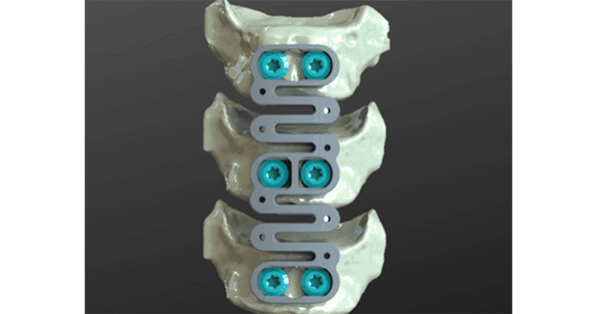
ReVivo Medical Spinal Plate
Trauma
Gramercy Extremity Orthopedics (GEO) completed its Series A financing, raising $9.8 million. Funds will be used to scale up technology infrastructure and continued product expansion with specific focus on the United States extremity market.
The company’s GEO CART is an industry-first, computerized, mobile implant and instrumentation inventory system enabled with RFID technology. No bigger than the average medical cart, the GEO CART system can hold over 1,000+ items tailored to the usage of a specific facility. The proprietary point-of-sale system reduces delays in surgery, decreases sterility risks to the patient, eliminates handwritten billing mistakes, automatically generates the Implant Usage Form, removes the cost to clean and sterilize instrumentation, and reduces facility operating expenses.
OsteoCentric Technologies secured $30 million in funding. The growth capital will fuel new trauma product introductions, and expand applications of Unifi Mechanical Integration (UnifiMI) technology across spine, joint reconstruction, sports, extremities, veterinary, limb salvage and dental implants.
UnifiMI was developed to address implant instability. Employing a proprietary method, OsteoCentric secures an implant to bone with mechanical integration. Proprietary thread geometry is capable of instantly interlocking with the bone to optimize acute and long-term implant stability.
WishBone Medical secured $10 million in growth capital financing.
The company’s implants and instruments in single-use, sterile-packed procedure kits address pediatric orthopedic applications. Its portfolio spans trauma, spine and sports medicine segments.
WishBone Medical will use the new capital to hire additional personnel and expedite product development while expanding the manufacturing facilities of its subsidiary, Red Star Contract Manufacturing. The strategic expenditures will be a means to broadening the company’s product portfolio and increasing inventory in support of sales.
Sports Medicine
Bioventus completed a strategic investment in Trice Medical, a company focused on developing and commercializing minimally invasive technologies for sports medicine and orthopedic surgical procedures. In conjunction with Bioventus leading the Series D funding round, the Bioventus will receive exclusive sales and distribution rights to Trice’s products outside of the U.S.
Trice combines their handheld arthroscope and portable ultrasound visualization technologies with differentiated surgical devices to treat a range of sports medicine and orthopedic conditions, including tendinopathy, planter fasciitis and carpel tunnel.
Embody secured $11.5 million in an oversubscribed equity financing and entered into a debt financing agreement to provide up to $9 million in term loans, of which an initial $5 million has been funded, for a total of $20.5 million in available funds.
Proceeds will support commercialization of Tapestry Biointegrative Implant, the launch of the Tapestry RCR system for rotator cuff repair and postmarket clinical studies of the Tapestry platform.
Embody’s Tapestry implant can be used in a broad range of tendon applications, including foot and ankle, knee and shoulder procedures.

Embody Tapestry RC Rotator Cuff Repair
Lazurite (then named Indago) closed a $10 million convertible note round of financing.
The funds will be used to complete pre-submission testing of the ArthroFree wireless camera system and submission for FDA clearance, as well as product development and intellectual property expansion. Product launch is expected in the first half of 2022.

Lazurite ArthroFree
Orthobiologics
CoreBone completed an investment round of $3.7 million. Funds will be used to extend CoreBone’s commercial global presence, primarily in China.
CoreBone develops and sells bioactive bone graft material for dental and orthopedic indications. It is the only company that uses cultured coral for bone grafting. Corals are grown in a closed, controlled aquatic system using proprietary technology and laboratory-made seawater enriched with bioactive nutrients and CoreBone’s own coral breeding source. The coral has similar properties to human or animal bones and replaces the need for using animal or human bone for bone grafting. This eliminates the risk of disease transfer associated with these products. In China, the import of foreign human-derived bone grafts is banned – creating a tremendous growth opportunity for CoreBone’s products.
Locate Bio raised an oversubscribed £10 million (USD ~$13.4 million) of equity investment. The investment brings the total funds raised by Locate Bio to over £18 million.
Proceeds will further advance the company’s pipeline of regenerative orthobiologic products, with key clinical and regulatory milestones expected to impact growth over the next two years.
The company’s lead product in development is called LDGraft, and is a low dose, controlled release rhBMP-2 for the treatment of degenerative disc disease.
The company received two breakthrough device designations from FDA at the start of 2021 for CognitOss and Chondro3, a biomimetic graft for osteochondral lesions. CognitOss is a single-stage therapy that combines the local delivery of therapeutically appropriate levels of antibiotics from a “smart material” that is designed to be responsive to the presence of infection and promote the regeneration of bone. It is designed to treat osteomyelitis, a debilitating inflammatory bone infection.
Robotics/Digital
Enhatch, developer of a cloud-based Intelligent Surgery platform, raised $9 million in a Series A round.
A key objective with this funding is to integrate the surgical platform into every aspect of the medical device industry. The ecosystem is an end-to-end technology platform delivering the next generation of smart surgical devices and enterprise workflow solutions.
Monogram Orthopedics has raised over $20 million and is currently raising up to $34 million in its active Series B round. Proceeds will help commercialize technology that meets the need for knee replacement technology that is well suited for active patient lifestyles, with minimized risk for additional surgeries down the line.
The company’s proprietary approach to total knee procedures will address issues including mechanical loosening, bone loss, dislocation, ease of revision and fracture. Using the Monogram software platform and product solution architecture, surgeons will be able to design optimized implants that improve stability and physiological loading.
Osso VR, a validated virtual reality (VR) surgical training and assessment platform, secured $27 million in Series B funding.
The technology provides on-demand, educational experiences that are repeatable and measurable to help surgeons reach proficiency with emerging surgical techniques and technologies. Osso VR works with companies like Johnson & Johnson, Stryker and Smith+Nephew, and houses the world’s largest surgical training library, offering 120+ modules in 10+ specialties.

Osso VR surgical training headset
Julie A. Vetalice is ORTHOWORLD’s Editorial Assistant.
Companies across all orthopedic market segments with products in development or recently cleared continue to secure funding to fuel commercialization efforts. We recap 18 funding announcements that took place since June 2021. Many of the companies are familiar names with previously-closed funding rounds.
Joint Replacement
Catalyst...
Companies across all orthopedic market segments with products in development or recently cleared continue to secure funding to fuel commercialization efforts. We recap 18 funding announcements that took place since June 2021. Many of the companies are familiar names with previously-closed funding rounds.
Joint Replacement
Catalyst OrthoScience raised $12.3 million in an oversubscribed Series D financing round.
Withing 2021, the shoulder replacement manufacturer has rebranded its product portfolio with the Archer family name, received FDA 510(k) clearance and began a limited launch for the Archer R1 Reverse Shoulder System, as well as introduced its Archer 3D Targeting imaging software for its current product offerings.
Additionally, the Archer CSR Total Shoulder System, Catalyst’s stemless, ellipsoid anatomic system, has continued rapid adoption and growth. Archer shoulder systems have been used in more than 3,000 surgeries.

Catalyst OrthoScience Archer R1 Reverse Shoulder
Garwood Medical Devices, developer of BioPrax technology to treat antibiotic-resistant bacterial biofilm infections associated with metallic orthopedic implants, completed a Series C round that closed at $4 million, as projected.
The company has now raised $11.4 million, including $3.8 million in the Series B round, which closed in late 2019. The company raised an initial $3.6 million in a Series A financing round, which closed in 2016. Funds raised will continue to support preclinical testing.
BioPrax is under investigation to study the elimination of biofilm infections on prosthetic knee implants during early intervention procedures, when used alongside the current standard of care.
BioPrax was accepted into FDA’s Breakthrough Device Program in 2019. The product is expected to be on the market in 2025.

Garwood Medical BioPrax Technology
MatOrtho received an investment of £6.7 million (~USD $9.4 million) to support its international growth and product development. The company produces knee, hip and finger joint replacement systems.
The funding will enable MatOrtho to accelerate international expansion into existing and new markets and will support research and development as the company continues to develop the next generation of implant technology.
Onkos Surgical raised $15 million in Series C funding.
The company markets implants and instruments that meet the needs of musculoskeletal tumor patients, with a focus on clinical challenges of implant loosening, soft tissue failures and infection.
Proceeds will drive revenue growth by accelerating the company’s research and development pipeline, expanding its commercial footprint and evolving manufacturing capabilities.
Spine
Empirical Spine closed a Series B financing of $10 million. Funds will advance the commercialization of the LimiFlex Paraspinous Tension Band, including completion of the ongoing pivotal trial and submitting all components of a premarket approval filing to FDA.
LimiFlex was developed to offer a minimally invasive means to stabilize the spine yet preserve motion.
The prospective, multicenter, controlled clinical trial is comparing LimiFlex Paraspinous Tension to transforaminal lumbar interbody fusion in the treatment of degenerative spondylolisthesis and lumbar spinal stenosis.
MiRus completed a highly oversubscribed $65 million funding round for growth of its spine and extremity portfolios and development of structural heart disease solutions using its proprietary Rhenium-based alloys (MoRe).
Implants made from Rhenium-based superalloys are reported to have superior mechanical strength, fatigue resistance and dramatically better biological performance vs. titanium, cobalt and nickel-based alloys used in medical implants.
ReVivo Medical completed its most recent equity offering.
Overall, the company has raised $2 million which will support completion of 50 surgical procedures in a clinical trial. Study participants will receive ReVivo Medical’s next-generation design anterior cervical plate and interbody cages used in anterior cervical discectomy and fusion procedures.

ReVivo Medical Spinal Plate
Trauma
Gramercy Extremity Orthopedics (GEO) completed its Series A financing, raising $9.8 million. Funds will be used to scale up technology infrastructure and continued product expansion with specific focus on the United States extremity market.
The company’s GEO CART is an industry-first, computerized, mobile implant and instrumentation inventory system enabled with RFID technology. No bigger than the average medical cart, the GEO CART system can hold over 1,000+ items tailored to the usage of a specific facility. The proprietary point-of-sale system reduces delays in surgery, decreases sterility risks to the patient, eliminates handwritten billing mistakes, automatically generates the Implant Usage Form, removes the cost to clean and sterilize instrumentation, and reduces facility operating expenses.
OsteoCentric Technologies secured $30 million in funding. The growth capital will fuel new trauma product introductions, and expand applications of Unifi Mechanical Integration (UnifiMI) technology across spine, joint reconstruction, sports, extremities, veterinary, limb salvage and dental implants.
UnifiMI was developed to address implant instability. Employing a proprietary method, OsteoCentric secures an implant to bone with mechanical integration. Proprietary thread geometry is capable of instantly interlocking with the bone to optimize acute and long-term implant stability.
WishBone Medical secured $10 million in growth capital financing.
The company’s implants and instruments in single-use, sterile-packed procedure kits address pediatric orthopedic applications. Its portfolio spans trauma, spine and sports medicine segments.
WishBone Medical will use the new capital to hire additional personnel and expedite product development while expanding the manufacturing facilities of its subsidiary, Red Star Contract Manufacturing. The strategic expenditures will be a means to broadening the company’s product portfolio and increasing inventory in support of sales.
Sports Medicine
Bioventus completed a strategic investment in Trice Medical, a company focused on developing and commercializing minimally invasive technologies for sports medicine and orthopedic surgical procedures. In conjunction with Bioventus leading the Series D funding round, the Bioventus will receive exclusive sales and distribution rights to Trice’s products outside of the U.S.
Trice combines their handheld arthroscope and portable ultrasound visualization technologies with differentiated surgical devices to treat a range of sports medicine and orthopedic conditions, including tendinopathy, planter fasciitis and carpel tunnel.
Embody secured $11.5 million in an oversubscribed equity financing and entered into a debt financing agreement to provide up to $9 million in term loans, of which an initial $5 million has been funded, for a total of $20.5 million in available funds.
Proceeds will support commercialization of Tapestry Biointegrative Implant, the launch of the Tapestry RCR system for rotator cuff repair and postmarket clinical studies of the Tapestry platform.
Embody’s Tapestry implant can be used in a broad range of tendon applications, including foot and ankle, knee and shoulder procedures.

Embody Tapestry RC Rotator Cuff Repair
Lazurite (then named Indago) closed a $10 million convertible note round of financing.
The funds will be used to complete pre-submission testing of the ArthroFree wireless camera system and submission for FDA clearance, as well as product development and intellectual property expansion. Product launch is expected in the first half of 2022.

Lazurite ArthroFree
Orthobiologics
CoreBone completed an investment round of $3.7 million. Funds will be used to extend CoreBone’s commercial global presence, primarily in China.
CoreBone develops and sells bioactive bone graft material for dental and orthopedic indications. It is the only company that uses cultured coral for bone grafting. Corals are grown in a closed, controlled aquatic system using proprietary technology and laboratory-made seawater enriched with bioactive nutrients and CoreBone’s own coral breeding source. The coral has similar properties to human or animal bones and replaces the need for using animal or human bone for bone grafting. This eliminates the risk of disease transfer associated with these products. In China, the import of foreign human-derived bone grafts is banned – creating a tremendous growth opportunity for CoreBone’s products.
Locate Bio raised an oversubscribed £10 million (USD ~$13.4 million) of equity investment. The investment brings the total funds raised by Locate Bio to over £18 million.
Proceeds will further advance the company’s pipeline of regenerative orthobiologic products, with key clinical and regulatory milestones expected to impact growth over the next two years.
The company’s lead product in development is called LDGraft, and is a low dose, controlled release rhBMP-2 for the treatment of degenerative disc disease.
The company received two breakthrough device designations from FDA at the start of 2021 for CognitOss and Chondro3, a biomimetic graft for osteochondral lesions. CognitOss is a single-stage therapy that combines the local delivery of therapeutically appropriate levels of antibiotics from a “smart material” that is designed to be responsive to the presence of infection and promote the regeneration of bone. It is designed to treat osteomyelitis, a debilitating inflammatory bone infection.
Robotics/Digital
Enhatch, developer of a cloud-based Intelligent Surgery platform, raised $9 million in a Series A round.
A key objective with this funding is to integrate the surgical platform into every aspect of the medical device industry. The ecosystem is an end-to-end technology platform delivering the next generation of smart surgical devices and enterprise workflow solutions.
Monogram Orthopedics has raised over $20 million and is currently raising up to $34 million in its active Series B round. Proceeds will help commercialize technology that meets the need for knee replacement technology that is well suited for active patient lifestyles, with minimized risk for additional surgeries down the line.
The company’s proprietary approach to total knee procedures will address issues including mechanical loosening, bone loss, dislocation, ease of revision and fracture. Using the Monogram software platform and product solution architecture, surgeons will be able to design optimized implants that improve stability and physiological loading.
Osso VR, a validated virtual reality (VR) surgical training and assessment platform, secured $27 million in Series B funding.
The technology provides on-demand, educational experiences that are repeatable and measurable to help surgeons reach proficiency with emerging surgical techniques and technologies. Osso VR works with companies like Johnson & Johnson, Stryker and Smith+Nephew, and houses the world’s largest surgical training library, offering 120+ modules in 10+ specialties.

Osso VR surgical training headset
Julie A. Vetalice is ORTHOWORLD’s Editorial Assistant.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.







