Copy to clipboard
Copy to clipboard 
Companies across orthopedics with products in development or recently cleared continue to secure funding to fuel commercialization efforts. We recap 13 funding announcements that took place in the first four months of 2021.
JOINT REPLACEMENT
Rejoint received a €3.8 Million (~USD $4.6 million) grant from the Smart Factory and Life Science National Grant Program, presented by the Italian Ministry of Economic Development.
Funds will support the company’s SMART BI-UNI KNEE project. Rejoint will apply the same manufacturing and technology concepts of its YourKnee TKA to the BI-UNI KNEE, including 3D printing, personalized implants and 3D preoperative planning and patient-specific instruments, assisted by computer and artificial intelligence (AI).
SPINE
ALM Ortho closed a seed round with investment from Paul DeJuliis, a private equity investor. Funds will support submission for FDA 510(k) clearance of their flagship Lateral Intervertebral Fusion Cage and SI Joint Fusion Device for both standard and patient-specific indications.
Partnering with supplier Amplify Additive, ALM Ortho seeks to combine current additive manufacturing technology with AI to create new advanced orthopedic implant products.
ALM Ortho believes that within the next five to 10 years, orthopedic implants coupled with preoperative surgical planning and design that leverages AI will become a minimum expectation from consumers. Future development plans include implants for the spine, total joints, oncology, CMF and extremities.
Axis Spine Technologies raised £2.2 million (~USD $3.1 million) in a funding round to support product launches of cleared devices, as well as new product development.
Axis is developing Anterior Spinal Implant technology which provides surgeons with increased correction and alignment options. The modular devices are inserted with less force than conventional implants to reduce the risks of structural damage which can lead to subsidence.
In 2020, the company received £830,000 (~USD $1.0 million) in new funding to bring products to the spine market, followed by FDA 510(k) clearance to market Axis-ALIF, their first implant system.
The funding will be used to support product launch of Axis-ALIF and development of oblique and lateral cage implants, as well as a highly differentiated access system.
Blue Ocean Spine, a Germany-based startup, received an investment from SHS. As an alternative to conventional spinal cages, Blue Ocean implants will permit surgeons to eliminate additional screw connections. Surgeons will also be freed from the need to maintain an inventory of a large number of implant variations in different sizes and dimensions.
This is the second collaboration for SHS and Blue Ocean’s founder, Guntmar Eisen, following a successful investment in EIT Emerging Implant Technologies which was acquired by Johnson & Johnson/DePuy Synthes in 2018.
Blue Ocean is developing functional and adaptable expandable cage systems, some of which require no additional screw.
Life Spine received an investment from Granite Creek Capital Partners. The infusion will enable Life Spine to aggressively pursue its growth initiatives through its Micro-Invasive platforms. The undisclosed investment was made from Granite Creek’s $200 million FlexCap II fund.
Granite Creek’s previous orthopedic industry investments include funding for Royal Biologics.
ReVivo Medical closed on a private equity offering, raising over $550,000. The company is developing elastic micro-motion implantable spinal devices. Results from an efficacy study demonstrated that loadsharing through elastic micro-motion may accelerate bone formation and interbody fusion.
ReVivo Medical recently received FDA-approval for a 50-patient clinical study to support its application for clearance of anterior cervical plates and interbody cages for commercial use. ReVivo intends to raise additional funds as the trial progresses.
ZygoFix completed a $2 million round. Funds will support further clinical studies, enhancements to the company’s implant and tools, and regulatory clearance.
ZygoFix’s zLOCK is a miniature screwless implant that provides spinal stability and fusion. zLOCK replaces complex screw stabilization, and patented bendable features enable adjustment to specific joints’ shapes during insertion.
The funding announcement was made alongside launch of an Israeli clinical study. An ongoing study in Hungary, which began in 2018, demonstrated meaningful and sustained reduction in pain and increase in quality of life for patients, all with a minimally invasive, simple and short procedure.
TRAUMA
OSSIO closed a $20 million financing deal to support aggressive growth plans. The funding comes as the company commenced U.S. launch of OSSIOfiber® Compression Screws for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis and bone grafts of the upper extremity, fibula, knee, ankle and foot in the presence of appropriate brace and/or immobilization.
The portfolio received FDA 510(k) market clearance in 2020. The initial product offering is applicable for use in the treatment of multiple lower and upper extremity injuries.
Comprising proprietary OSSIOfiber Intelligent Bone Regeneration Technology, the screws are engineered to achieve the optimal balance of flexural, torque, axial and shear strength.
ORTHOBIOLOGICS
Amplicore closed its series seed round of funding at $4 million. Proceeds will allow the company to complete preclinical development of its two leading products, AM3101 and AM1101 injectables, and position the company for the next round of financing.
Amplicore is pursuing a regenerative approach to treat joint osteoarthritis, cartilage damage, degenerative disc disease and acute meniscus tear. AM1101 is in development as a treatment for osteoarthritis designed to reduce pain, inhibit cartilage degradation and promote tissue regeneration of aberrant cartilage.
Amplicore is also leveraging a study on the healing of acute meniscal tear after surgical repair, supported by a grant from the U.S. Department of Defense. AM3101 technology has demonstrated promising preclinical results as a potential minimally invasive and regenerative approach to managing soft tissue injury.
GreenBone Ortho completed a €10 million (USD ~$11.9 million) Series B investment round, bringing the total raised to €22 million (~$26.3 million) since 2015.
GreenBone specializes in bone regeneration using a proprietary 3D scaffold made of natural wood-derived materials. Its highly porous graft has an optimal biomaterial surface area of resorbable nano-crystalline hydroxyapatite and beta-tricalcium phosphate plus magnesium and strontium ions to mimic natural bone chemistry, morphology and 3D architecture.
The product was approved under the CE Mark in 2019 for a portfolio of solutions in a variety of orthopedic applications including large segmental bone defects.
The new funding will support new product developments for indications like spinal and maxillofacial surgery. The funds will also help build a new production line, and market launch of the approved product.
RevBio was awarded a two-year, $2 million Phase II Small Business Innovation Research to pursue the treatment of wrist fractures with its Tetranite bone adhesive technology. The injectable adhesive requires only a small incision to deliver the material, making it a more minimally invasive treatment option than standard open surgery.
Other applications in various stages of development for the technology include spinal fusion, joint reconstruction, suture anchor attachment, animal health, etc.
DIGITAL TOOLS
Canary Medical received a $20 million venture loan facility for general working capital purposes. The company has developed the Canary Health Implantable Reporting Processor (CHIRP), which uses sensor and novel communication technology to provide real-time monitoring and feedback. The CHIRP sensor is modular, and can be customized and embedded in the tibial extension segment of a commercial knee prosthesis. The sensor is powered by a pacemaker battery with a low draw that enables operation for 20 years or more, matching most implants’ lifespan.
Using gyroscopes, accelerometers and a pedometer, CHIRP generates clinically relevant data on device function, range of motion, gait, patient behavior and early signs of loosening/infection/failure.
Canary’s lead program addresses the primary total knee joint replacement market, and the company is partnered with Zimmer Biomet for use with the Persona-IQ knee. FDA granted Canary a Breakthrough Device Designation in October 2019, and product launch could occur within 2021.
CHIRP’s modular design allows it to be customized for a range of orthopedic devices in the future, including total knees from other manufacturers, total hip and total shoulder replacements and fracture fixation devices, potentially in 2023 and beyond.
Canary Medical has previously raised approximately $45 million from a syndicate of investors.
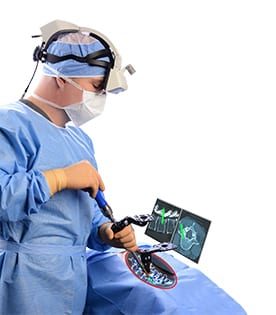
Augmedics raised $36 million in an oversubscribed Series C financing. This comes eight months after the company closed a $21 million Series B, bringing the total funding to date to $63 million.
Funds will support continued U.S. commercialization and development in ex-U.S. markets for the xvision Spine system, as well as next-gen products, new indications and a CE Mark.
Augmedics’ xvision Spine (XVS) is the first and only FDA-cleared augmented reality surgical image guidance system. XVS allows surgeons to visualize a patient’s 3D spinal anatomy during surgery as if they had “x-ray vision,” and to navigate instruments and implants while looking directly at the patient.
Following a 1Q20 launch, Augmedics began U.S. sales of XVS in 2H20 and closed the year with over 250 spinal surgeries performed.
Julie A. Vetalice is ORTHOWORLD’s Editorial Assistant.
Companies across orthopedics with products in development or recently cleared continue to secure funding to fuel commercialization efforts. We recap 13 funding announcements that took place in the first four months of 2021.
JOINT REPLACEMENT
Rejoint received a €3.8 Million (~USD $4.6 million) grant from the Smart Factory and Life...
Companies across orthopedics with products in development or recently cleared continue to secure funding to fuel commercialization efforts. We recap 13 funding announcements that took place in the first four months of 2021.
JOINT REPLACEMENT
Rejoint received a €3.8 Million (~USD $4.6 million) grant from the Smart Factory and Life Science National Grant Program, presented by the Italian Ministry of Economic Development.
Funds will support the company’s SMART BI-UNI KNEE project. Rejoint will apply the same manufacturing and technology concepts of its YourKnee TKA to the BI-UNI KNEE, including 3D printing, personalized implants and 3D preoperative planning and patient-specific instruments, assisted by computer and artificial intelligence (AI).
SPINE
ALM Ortho closed a seed round with investment from Paul DeJuliis, a private equity investor. Funds will support submission for FDA 510(k) clearance of their flagship Lateral Intervertebral Fusion Cage and SI Joint Fusion Device for both standard and patient-specific indications.
Partnering with supplier Amplify Additive, ALM Ortho seeks to combine current additive manufacturing technology with AI to create new advanced orthopedic implant products.
ALM Ortho believes that within the next five to 10 years, orthopedic implants coupled with preoperative surgical planning and design that leverages AI will become a minimum expectation from consumers. Future development plans include implants for the spine, total joints, oncology, CMF and extremities.
Axis Spine Technologies raised £2.2 million (~USD $3.1 million) in a funding round to support product launches of cleared devices, as well as new product development.
Axis is developing Anterior Spinal Implant technology which provides surgeons with increased correction and alignment options. The modular devices are inserted with less force than conventional implants to reduce the risks of structural damage which can lead to subsidence.
In 2020, the company received £830,000 (~USD $1.0 million) in new funding to bring products to the spine market, followed by FDA 510(k) clearance to market Axis-ALIF, their first implant system.
The funding will be used to support product launch of Axis-ALIF and development of oblique and lateral cage implants, as well as a highly differentiated access system.
Blue Ocean Spine, a Germany-based startup, received an investment from SHS. As an alternative to conventional spinal cages, Blue Ocean implants will permit surgeons to eliminate additional screw connections. Surgeons will also be freed from the need to maintain an inventory of a large number of implant variations in different sizes and dimensions.
This is the second collaboration for SHS and Blue Ocean’s founder, Guntmar Eisen, following a successful investment in EIT Emerging Implant Technologies which was acquired by Johnson & Johnson/DePuy Synthes in 2018.
Blue Ocean is developing functional and adaptable expandable cage systems, some of which require no additional screw.
Life Spine received an investment from Granite Creek Capital Partners. The infusion will enable Life Spine to aggressively pursue its growth initiatives through its Micro-Invasive platforms. The undisclosed investment was made from Granite Creek’s $200 million FlexCap II fund.
Granite Creek’s previous orthopedic industry investments include funding for Royal Biologics.
ReVivo Medical closed on a private equity offering, raising over $550,000. The company is developing elastic micro-motion implantable spinal devices. Results from an efficacy study demonstrated that loadsharing through elastic micro-motion may accelerate bone formation and interbody fusion.
ReVivo Medical recently received FDA-approval for a 50-patient clinical study to support its application for clearance of anterior cervical plates and interbody cages for commercial use. ReVivo intends to raise additional funds as the trial progresses.
ZygoFix completed a $2 million round. Funds will support further clinical studies, enhancements to the company’s implant and tools, and regulatory clearance.
ZygoFix’s zLOCK is a miniature screwless implant that provides spinal stability and fusion. zLOCK replaces complex screw stabilization, and patented bendable features enable adjustment to specific joints’ shapes during insertion.
The funding announcement was made alongside launch of an Israeli clinical study. An ongoing study in Hungary, which began in 2018, demonstrated meaningful and sustained reduction in pain and increase in quality of life for patients, all with a minimally invasive, simple and short procedure.
TRAUMA
OSSIO closed a $20 million financing deal to support aggressive growth plans. The funding comes as the company commenced U.S. launch of OSSIOfiber® Compression Screws for maintenance of alignment and fixation of bone fractures, comminuted fractures, fragments, osteotomies, arthrodesis and bone grafts of the upper extremity, fibula, knee, ankle and foot in the presence of appropriate brace and/or immobilization.
The portfolio received FDA 510(k) market clearance in 2020. The initial product offering is applicable for use in the treatment of multiple lower and upper extremity injuries.
Comprising proprietary OSSIOfiber Intelligent Bone Regeneration Technology, the screws are engineered to achieve the optimal balance of flexural, torque, axial and shear strength.
ORTHOBIOLOGICS
Amplicore closed its series seed round of funding at $4 million. Proceeds will allow the company to complete preclinical development of its two leading products, AM3101 and AM1101 injectables, and position the company for the next round of financing.
Amplicore is pursuing a regenerative approach to treat joint osteoarthritis, cartilage damage, degenerative disc disease and acute meniscus tear. AM1101 is in development as a treatment for osteoarthritis designed to reduce pain, inhibit cartilage degradation and promote tissue regeneration of aberrant cartilage.
Amplicore is also leveraging a study on the healing of acute meniscal tear after surgical repair, supported by a grant from the U.S. Department of Defense. AM3101 technology has demonstrated promising preclinical results as a potential minimally invasive and regenerative approach to managing soft tissue injury.
GreenBone Ortho completed a €10 million (USD ~$11.9 million) Series B investment round, bringing the total raised to €22 million (~$26.3 million) since 2015.
GreenBone specializes in bone regeneration using a proprietary 3D scaffold made of natural wood-derived materials. Its highly porous graft has an optimal biomaterial surface area of resorbable nano-crystalline hydroxyapatite and beta-tricalcium phosphate plus magnesium and strontium ions to mimic natural bone chemistry, morphology and 3D architecture.
The product was approved under the CE Mark in 2019 for a portfolio of solutions in a variety of orthopedic applications including large segmental bone defects.
The new funding will support new product developments for indications like spinal and maxillofacial surgery. The funds will also help build a new production line, and market launch of the approved product.
RevBio was awarded a two-year, $2 million Phase II Small Business Innovation Research to pursue the treatment of wrist fractures with its Tetranite bone adhesive technology. The injectable adhesive requires only a small incision to deliver the material, making it a more minimally invasive treatment option than standard open surgery.
Other applications in various stages of development for the technology include spinal fusion, joint reconstruction, suture anchor attachment, animal health, etc.
DIGITAL TOOLS
Canary Medical received a $20 million venture loan facility for general working capital purposes. The company has developed the Canary Health Implantable Reporting Processor (CHIRP), which uses sensor and novel communication technology to provide real-time monitoring and feedback. The CHIRP sensor is modular, and can be customized and embedded in the tibial extension segment of a commercial knee prosthesis. The sensor is powered by a pacemaker battery with a low draw that enables operation for 20 years or more, matching most implants’ lifespan.
Using gyroscopes, accelerometers and a pedometer, CHIRP generates clinically relevant data on device function, range of motion, gait, patient behavior and early signs of loosening/infection/failure.
Canary’s lead program addresses the primary total knee joint replacement market, and the company is partnered with Zimmer Biomet for use with the Persona-IQ knee. FDA granted Canary a Breakthrough Device Designation in October 2019, and product launch could occur within 2021.
CHIRP’s modular design allows it to be customized for a range of orthopedic devices in the future, including total knees from other manufacturers, total hip and total shoulder replacements and fracture fixation devices, potentially in 2023 and beyond.
Canary Medical has previously raised approximately $45 million from a syndicate of investors.
Augmedics raised $36 million in an oversubscribed Series C financing. This comes eight months after the company closed a $21 million Series B, bringing the total funding to date to $63 million.
Funds will support continued U.S. commercialization and development in ex-U.S. markets for the xvision Spine system, as well as next-gen products, new indications and a CE Mark.
Augmedics’ xvision Spine (XVS) is the first and only FDA-cleared augmented reality surgical image guidance system. XVS allows surgeons to visualize a patient’s 3D spinal anatomy during surgery as if they had “x-ray vision,” and to navigate instruments and implants while looking directly at the patient.
Following a 1Q20 launch, Augmedics began U.S. sales of XVS in 2H20 and closed the year with over 250 spinal surgeries performed.
Julie A. Vetalice is ORTHOWORLD’s Editorial Assistant.

You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.