





Paragon 28 received FDA 510(k) clearance to market the Phantom® ActivCore Nail, indicated for tibio-talo-calcaneal arthrodesis. Full commercial launch is slated for 2Q20, representing the second of three hindfoot fusion nailing systems that the company plans to introduce this year.
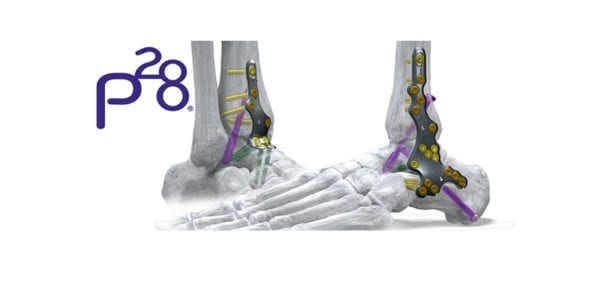
ActivCore features an outer spring sheath and inner sliding core to support consistent active compression and up to 8mm of bone resorption across all joint spaces. The nail accommodates two 7.2 mm calcaneal pegs that are inserted from posterior to anterior at a variable angle.
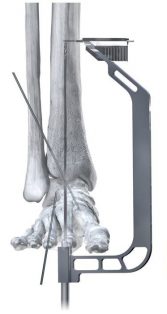
PRECISION® Guide technology supports reproducibility when placing a 3.0 mm drill-pin from distal to proximal, through the center of the calcaneus and up to the tibia. A flex coil design in the proximal end affords flexibility as the implant is set through the contouring of the tibial canal. Pegs are inserted from posterior to anterior and medial to lateral, eliminating the need to take down the fibula.
Phantom ActivCore Nails are made from Type II Anodized Titanium Alloy and will be offered in 22 size options with three diameters and four lengths. The system is implanted with the carbon fiber Phantom® Ghost™ Outrigger. Radiolucent and inlay features of Outrigger allow for visualization of peg trajectory under fluoroscopy prior to drilling.
Paragon 28 received FDA 510(k) clearance to market the Phantom® ActivCore Nail, indicated for tibio-talo-calcaneal arthrodesis. Full commercial launch is slated for 2Q20, representing the second of three hindfoot fusion nailing systems that the company plans to introduce this year.
ActivCore features an outer spring sheath and inner...
Paragon 28 received FDA 510(k) clearance to market the Phantom® ActivCore Nail, indicated for tibio-talo-calcaneal arthrodesis. Full commercial launch is slated for 2Q20, representing the second of three hindfoot fusion nailing systems that the company plans to introduce this year.
ActivCore features an outer spring sheath and inner sliding core to support consistent active compression and up to 8mm of bone resorption across all joint spaces. The nail accommodates two 7.2 mm calcaneal pegs that are inserted from posterior to anterior at a variable angle.
PRECISION® Guide technology supports reproducibility when placing a 3.0 mm drill-pin from distal to proximal, through the center of the calcaneus and up to the tibia. A flex coil design in the proximal end affords flexibility as the implant is set through the contouring of the tibial canal. Pegs are inserted from posterior to anterior and medial to lateral, eliminating the need to take down the fibula.
Phantom ActivCore Nails are made from Type II Anodized Titanium Alloy and will be offered in 22 size options with three diameters and four lengths. The system is implanted with the carbon fiber Phantom® Ghost™ Outrigger. Radiolucent and inlay features of Outrigger allow for visualization of peg trajectory under fluoroscopy prior to drilling.


You are out of free articles for this month
Subscribe as a Guest for $0 and unlock a total of 5 articles per month.
You are out of five articles for this month
Subscribe as an Executive Member for access to unlimited articles, THE ORTHOPAEDIC INDUSTRY ANNUAL REPORT and more.
JV
Julie Vetalice is ORTHOWORLD's Editorial Assistant. She has covered the orthopedic industry for over 20 years, having joined the company in 1999.